Effect of Crystallinity on the Field Emission Characteristics of Carbon Nanotube Grown on W-Co Bimetallic Catalyst
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
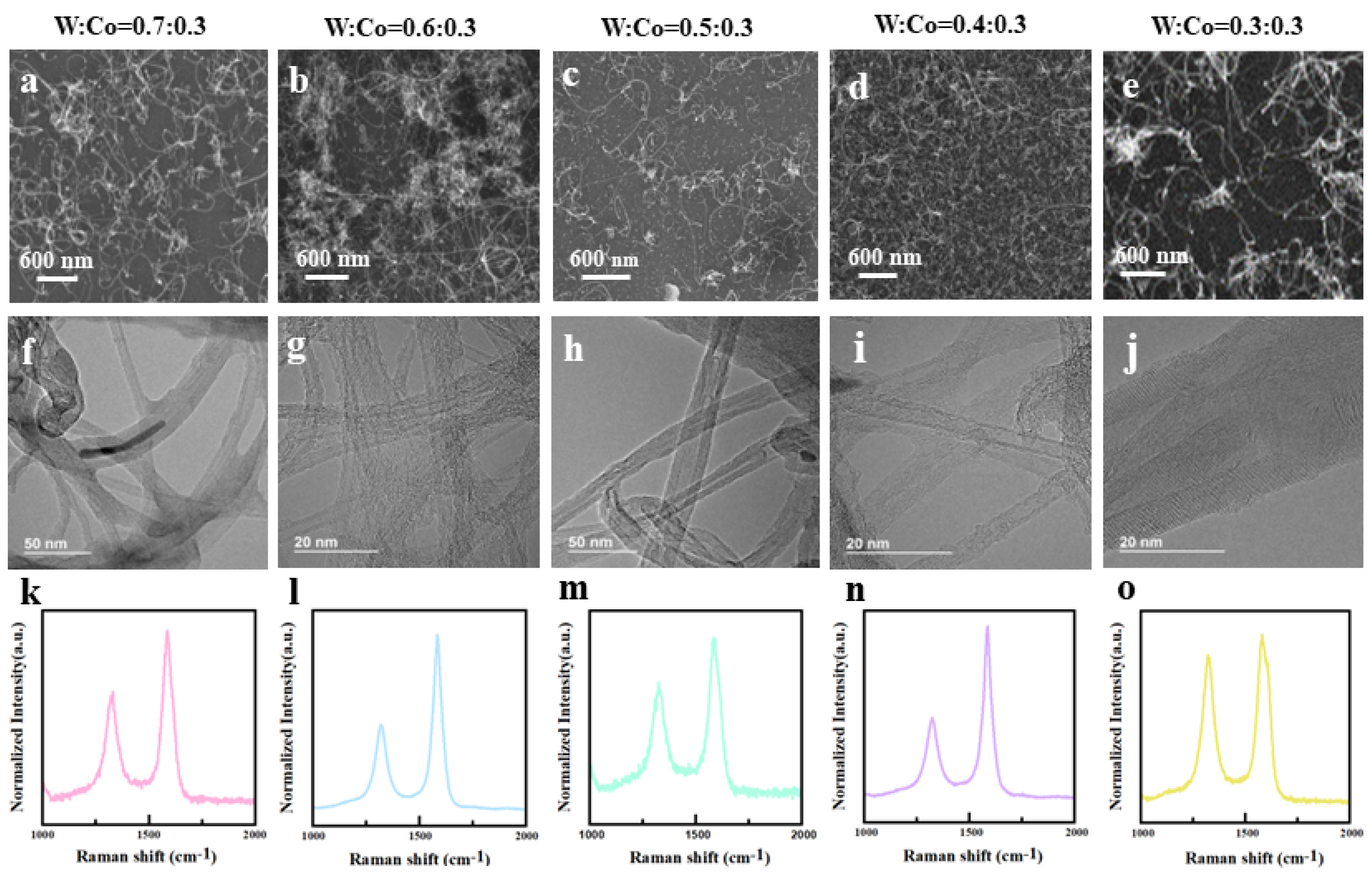
3.1. Synthesis and Charcterization of CNTs Grown on W-Co Catalyst
3.2. Field Emission Characterization of CNTs Grown on W-Co Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giubileo, F.; Di Bartolomeo, A.; Iemmo, L.; Luongo, G.; Urban, F. Field Emission from Carbon Nanostructures. Appl. Sci. 2018, 8, 526. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Yeow, J. Nanotube field electron emission: Principles, development, and applications. Nanotechnology 2015, 26, 242001. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Lee, S.H.; Go, H.; Kim, S.J.; Noh, J.H.; Lee, C.J. High-performance cold cathode X-ray tubes using a carbon nanotube field electron emitter. ACS Nano 2022, 16, 10231–10241. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- De Jonge, N. Carbon nanotube electron sources for electron microscopes. Adv. Imaging Electron Phys. 2009, 156, 203–233. [Google Scholar]
- Jagadeesan, A.K.; Thangavelu, K.; Dhananjeyan, V. Carbon Nanotubes: Synthesis, Properties and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Wang, X.; Ding, F. How a Solid Catalyst Determines the Chirality of the Single-Wall Carbon Nanotube Grown on It. J. Phys. Chem. Lett. 2019, 10, 735–741. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Qi, K.; Yang, J.; Xu, Z.; Li, M.; Zhao, X.; Bai, X.; Li, Y. Growing Zigzag (16,0) Carbon Nanotubes with Structure-Defined Catalysts. J. Am. Chem. Soc. 2015, 137, 8688–8691. [Google Scholar] [CrossRef] [PubMed]
- Reich, S.; Li, L.; Robertson, J. Control the chirality of carbon nanotubes by epitaxial growth. Chem. Phys. Lett. 2006, 421, 469–472. [Google Scholar] [CrossRef]
- Liang, S.; Huang, N.Y.; Deng, S.Z.; Xu, N.S. Chiral and quantum size effects of single-wall carbon nanotubes on field emission. Appl. Phys. Lett. 2004, 85, 813–815. [Google Scholar] [CrossRef]
- Shiratori, Y.; Furuichi, K.; Noda, S.; Sugime, H.; Tsuji, Y.; Zhang, Z.; Maruyama, S.; Yamaguchi, Y. Field Emission Properties of Single-Walled Carbon Nanotubes with a Variety of Emitter Morphologies. Jpn. J. Appl. Phys. 2008, 47, 4780. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Dai, Y.; Wang, C. Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem. Eng. J. 2021, 408, 127268. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, S.; Yang, F.; Li, Y. Atomic-scale evidence of catalyst evolution for the structure-controlled growth of single-walled carbon nanotubes. Acc. Chem. Res. 2022, 55, 3334–3344. [Google Scholar] [CrossRef]
- Nessim, G.D.; Hart, A.J.; Kim, J.S.; Acquaviva, D.; Oh, J.; Morgan, C.D.; Seita, M.; Leib, J.S.; Thompson, C.V. Tuning of vertically-aligned carbon nanotube diameter and areal density through catalyst pre-treatment. Nano Lett. 2008, 8, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Kibria, A.K.M.F.; Shajahan, M.; Mo, Y.H.; Kim, M.J.; Nahm, K.S. Long activity of Co-Mo/MgO catalyst for the synthesis of carbon nanotubes in large-scale and application feasibility of the grown tubes. Diam. Relat. Mater. 2004, 13, 1865–1872. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Pfefferle, L.D.; Haller, G.L. Pt-Co Bimetallic Catalyst Supported on Single-Walled Carbon Nanotubes: Effect of Alloy Formation and Oxygen Containing Groups. J. Phys. Chem. C 2010, 114, 16996–17002. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Si, J.; Zhao, X.; Qi, K.; Jin, C.; Zhang, Z.; Li, M.; Zhang, D.; Yang, J.; et al. Water-Assisted Preparation of High-Purity Semiconducting (14,4) Carbon Nanotubes. ACS Nano 2017, 11, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.; Xu, Z.; Peng, F. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- Herrera, J.E.; Resasco, D.E. Role of Co-W Interaction in the Selective Growth of Single-Walled Carbon Nanotubes from CO Disproportionation. J. Phys. Chem. B 2003, 107, 3738–3746. [Google Scholar] [CrossRef]
- Thapa, A.; Jungjohann, K.L.; Wang, X.; Li, W. Improving field emission properties of vertically aligned carbon nanotube arrays through a structure modification. J. Mater. Sci. 2020, 55, 2101–2117. [Google Scholar] [CrossRef]
- Singh, B.K.; Cho, S.; Bartwal, K.S.; Hoa, N.D.; Ryu, H. Synthesis of MWNTs using Fe–Mo bimetallic catalyst by CVD method for field emission application. Solid State Commun. 2007, 144, 498–502. [Google Scholar] [CrossRef]
- Tang, H.; Liu, R.; Huang, W.; Zhu, W.; Qian, W.; Dong, C. Field Emission of Multi-Walled Carbon Nanotubes from Pt-Assisted Chemical Vapor Deposition. Nanomaterials 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, F.; Chen, J.; Ding, L.; Liu, X.; Yao, F.; Li, M.; Zhang, D.; Zhang, Z.; Liu, X.; et al. Selective growth of chirality-enriched semiconducting carbon nanotubes by using bimetallic catalysts from salt precursors. Nanoscale 2018, 10, 6922–6927. [Google Scholar] [CrossRef]
- ICDD. Powder Diffraction File. PDF-2/Release 2009; International Centre for Diffraction Data: Newtown Square, PA, USA, 2009. [Google Scholar]
- Awadallah, A.E.; Aboul-Enein, A.A. Catalytic decomposition of methane to COx-free hydrogen and carbon nanotubes over Co-W/MgO catalysts. Egypt. J. Pet. 2015, 24, 299–306. [Google Scholar] [CrossRef]
- Podenok, S.; Sveningsson, M.; Hansen, K.; Campbell, E.E.B. Electric field enhancement factors around a metallic, end-capped cylinder. Nano 2006, 1, 87–93. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakato, Y.; Murakoshi, K. Absolute potential of the Fermi level of isolated single-walled carbon nanotubes. Phys. Rev. B 2003, 68, 35434. [Google Scholar] [CrossRef]
- Shimoi, N. Effect of increased crystallinity of single-walled carbon nanotubes used as field emitters on their electrical properties. J. Appl. Phys. 2015, 118, 214304. [Google Scholar] [CrossRef]
- Yasuhito Gotoh, Y.G.; Masayoshi Nagao, M.N.; Motoki Matsubara, M.M.; Kazunori Inoue, K.I.; Hiroshi Tsuji, H.T.; Junzo Ishikawa, J.I. Relationship between Effective Work Functions and Noise Powers of Emission Currents in Nickel-Deposited Field Emitters. Jpn. J. Appl. Phys. 1996, 35, L1297. [Google Scholar] [CrossRef]
- Ishikawa, J.; Tsuji, H.; Inoue, K.; Nagao, M.; Sasaki, T.; Kaneko, T.; Gotoh, Y. Estimation of metal-deposited field emitters for the micro vacuum tube. Jpn. J. Appl. Phys. 1993, 32, L342–L345. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X.D.; Wang, E.G.; Wang, Z.L. Field emission of individual carbon nanotube with in situ tip image and real work function. Appl. Phys. Lett. 2005, 87, 163106. [Google Scholar] [CrossRef]
- Maity, P.C.; Gandhi, S.; Dixit, M.; Lahiri, I. Systematic growth of carbon nanotubes on aluminum substrate for enhanced field emission performance. J. Vac. Sci. Technol. B 2021, 39, 022801. [Google Scholar]
- Huang, L.; Lau, S.P.; Zhang, Y.B.; Tay, B.K.; Fu, Y.Q. The synthesis of carbon nanotubes and zirconium carbide composite films on a glass substrate. Nanotechnology 2004, 15, 663. [Google Scholar] [CrossRef]
- Shang, N.G.; Tan, Y.Y.; Stolojan, V.; Papakonstantinou, P.; Silva, S. High-rate low-temperature growth of vertically aligned carbon nanotubes. Nanotechnology 2010, 21, 505604. [Google Scholar] [CrossRef]





| ID/IG | Catalyst Treatment Temperature/°C | Catalyst Treatment Atmosphere | CNT Growth Temperature/°C | CNT Growth Atmosphere |
|---|---|---|---|---|
| 0.23 | 750 | Ar:H2 = 290:100 sccm | 800 | Ar:H2:C2H4 = 330:500:100 sccm |
| 0.36 | 800 | Ar:H2 = 290:100 sccm | 800 | Ar:H2:C2H4 = 330:500:100 sccm |
| 0.40 | 800 | Ar:H2 = 500 sccm | 800 | Ar:H2:C2H4 = 330:500:100 sccm |
| 0.49 | 750 | Ar:H2 = 290:100 sccm | 750 | Ar:H2:C2H4 = 330:500:100 sccm |
| 0.65 | 750 | Ar:H2 = 500 sccm | 750 | Ar:H2:C2H4 = 330:500:100 sccm |
| Catalyst | Jmax (mA/cm2) | Turn-on Field (V/μm) | Reference |
|---|---|---|---|
| W-Co | 6 | 0.45 | This work |
| Fe-Mo | 1.16 | 2 | [22] |
| Co-Mo | 1.14 | 3.17 | [16] |
| Ni-Co | 2.17 | >2.5 | [33] |
| Zr-Fe | \ | 3.2 | [34] |
| Fe-Ti | \ | 13.9 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Q.; Wu, Y.; Song, G.; Xu, Z.; Ke, Y.; Zhan, R.; Chen, J.; Zhang, Y.; Deng, S. Effect of Crystallinity on the Field Emission Characteristics of Carbon Nanotube Grown on W-Co Bimetallic Catalyst. Nanomaterials 2024, 14, 819. https://doi.org/10.3390/nano14100819
Yao Q, Wu Y, Song G, Xu Z, Ke Y, Zhan R, Chen J, Zhang Y, Deng S. Effect of Crystallinity on the Field Emission Characteristics of Carbon Nanotube Grown on W-Co Bimetallic Catalyst. Nanomaterials. 2024; 14(10):819. https://doi.org/10.3390/nano14100819
Chicago/Turabian StyleYao, Qi, Yiting Wu, Guichen Song, Zhaoyin Xu, Yanlin Ke, Runze Zhan, Jun Chen, Yu Zhang, and Shaozhi Deng. 2024. "Effect of Crystallinity on the Field Emission Characteristics of Carbon Nanotube Grown on W-Co Bimetallic Catalyst" Nanomaterials 14, no. 10: 819. https://doi.org/10.3390/nano14100819





