Boron and Nitrogen Co-Doped Porous Graphene Nanostructures for the Electrochemical Detection of Poisonous Heavy Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
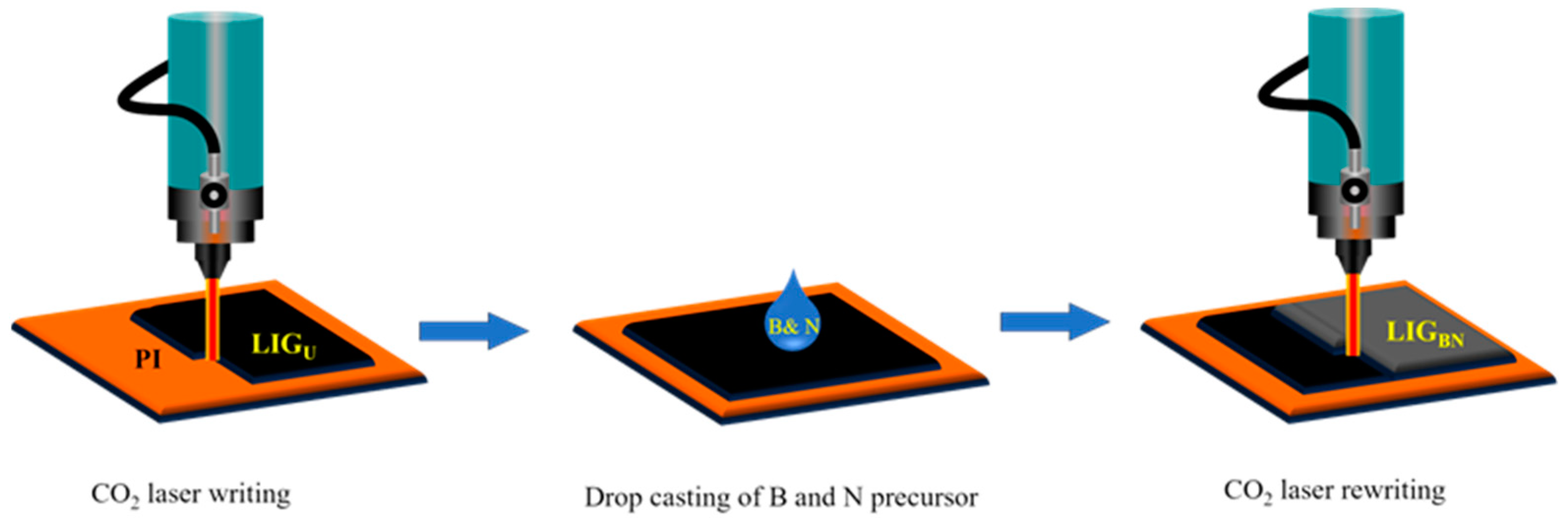
2.2. Fabrication of Boron and Nitrogen Co-Doped Laser-Induced Graphene
2.3. Characterisation
2.4. Electrochemical Measurements
3. Results and Discussions
3.1. Morphological and Microstructural Studies
3.2. Raman Analysis
3.3. XPS Analysis
3.4. Electrochemical Characterisation
3.5. Electrochemical Impedance Spectroscopy Studies
3.6. Optimisation of the Electrochemical Parameters
3.7. Electrochemical Detection of Pb2+ and Cd2+ Using SWV
3.7.1. Individual Detection of Pb2+ Using SWV
3.7.2. Individual Detection of Cd2+ Using SWV
3.7.3. Simultaneous Detection Cd2+ and Pb2+ Using SWV
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, 04691. [Google Scholar] [CrossRef]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy Metal Pollution: Source, Impact, and Remedies. In Biomanagement of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2011; pp. 1–28. [Google Scholar]
- Rama Jyothi, N. Heavy Metal Sources and Their Effects on Human Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Trzcinka-Ochocka, M.; Brodzka, R.; Janasik, B. Useful and Fast Method for Blood Lead and Cadmium Determination Using ICP-MS and GF-AAS; Validation Parameters. J. Clin. Lab. Anal. 2016, 30, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Li, X.; Jia, H.; Yu, X.; Wang, Z.; Yao, J.; Mao, X. Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules 2022, 27, 8176. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, B.; Leal, L.O.; Ferrer, L.; Cerdà, V. Determination of Lead by Atomic Fluorescence Spectrometry Using an Automated Extraction/Pre-Concentration Flow System. J. Anal. At. Spectrom. 2015, 30, 1072–1079. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Liu, G.; Thi Dieu Thuy, N. A Disposable and Flexible Electrochemical Sensor for the Sensitive Detection of Heavy Metals Based on a One-Step Laser-Induced Surface Modification: A New Strategy for the Batch Fabrication of Sensors. Sens. Actuators B Chem. 2022, 350, 130834. [Google Scholar] [CrossRef]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-Wave Voltammetry. ChemTexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Yen, Y.K.; Yan, W.M.; Huang, G.W. Laser-Scribed Graphene Electrodes Functionalized with Nafion/Fe3O4 Nanohybrids for the Ultrasensitive Detection of Neurotoxin Drug Clioquinol. ACS Omega 2022, 7, 15936–15950. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Yu, Y. Graphene-Derived Nanomaterials as Recognition Elements for Electrochemical Determination of Heavy Metal Ions: A Review. Microchim. Acta 2019, 186, 171. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Mathur, D. Graphene-Based Nanocomposites as Sensing Elements for the Electrochemical Detection of Pesticides: A Review. J. Solid State Electrochem. 2021, 25, 2145–2159. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef] [PubMed]
- Kumunda, C.; Adekunle, A.S.; Mamba, B.B.; Hlongwa, N.W.; Nkambule, T.T.I. Electrochemical Detection of Environmental Pollutants Based on Graphene Derivatives: A Review. Front. Mater. 2021, 7, 616787. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Ray, A.; Roth, J.; Saruhan, B. Laser-Induced Interdigital Structured Graphene Electrodes Based Flexible Micro-Supercapacitor for Efficient Peak Energy Storage. Molecules 2022, 27, 329. [Google Scholar] [CrossRef]
- Raza, T.; Tufail, M.K.; Ali, A.; Boakye, A.; Qi, X.; Ma, Y.; Ali, A.; Qu, L.; Tian, M. Wearable and Flexible Multifunctional Sensor Based on Laser-Induced Graphene for the Sports Monitoring System. ACS Appl. Mater. Interfaces 2022, 14, 54170–54181. [Google Scholar] [CrossRef]
- Lopes, D.V.; Santos, N.F.; Moura, J.P.; Fernandes, A.J.S.; Costa, F.M.; Kovalevsky, A.V. Design of Laser-Induced Graphene Electrodes for Water Splitting. Int. J. Hydrogen Energy 2023, 48, 4158–4172. [Google Scholar] [CrossRef]
- Lin, X.; Lu, Z.; Dai, W.; Liu, B.; Zhang, Y.; Li, J.; Ye, J. Laser Engraved Nitrogen-Doped Graphene Sensor for the Simultaneous Determination of Cd(II) and Pb(II). J. Electroanal. Chem. 2018, 828, 41–49. [Google Scholar] [CrossRef]
- Jeong, S.; Yang, S.; Lee, Y.J.; Lee, S.H. Laser-Induced Graphene Incorporated with Silver Nanoparticles Applied for Heavy Metal Multi-Detection. J. Mater. Chem. A Mater. 2023, 11, 13409–13418. [Google Scholar] [CrossRef]
- Saisree, S.; Arya Nair, J.S.; Karunakaran Yesodha, S. Graphene Quantum Dots Doped with Sulfur and Nitrogen as Versatile Electrochemical Sensors for Heavy Metal Ions Cd(II), Pb(II), and Hg(II). ACS Appl. Nano Mater. 2023, 6, 1224–1234. [Google Scholar] [CrossRef]
- Nandee, R.; Chowdhury, M.A.; Shahid, A.; Hossain, N.; Rana, M. Band Gap Formation of 2D Materialin Graphene: Future Prospect and Challenges. Results Eng. 2022, 15, 100474. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom Doped Graphene Engineering for Energy Storage and Conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Pumera, M. Heteroatom Modified Graphenes: Electronic and Electrochemical Applications. J. Mater. Chem. C Mater. 2014, 2, 6454–6461. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible Boron-Doped Laser-Induced Graphene Microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, C.; Li, N.; Zhang, S.; Song, Y.; Chen, L.; Xi, M.; Yu, X.; Wang, W.; Kong, M.; et al. One-Step Fabrication of Nitrogen-Doped Laser-Induced Graphene Derived from Melamine/Polyimide for Enhanced Flexible Supercapacitors. CrystEngComm 2022, 24, 1866–1876. [Google Scholar] [CrossRef]
- Khandelwal, M.; Van Tran, C.; Lee, J.; Bin In, J. Nitrogen and Boron Co-Doped Densified Laser-Induced Graphene for Supercapacitor Applications. Chem. Eng. J. 2021, 428, 131119. [Google Scholar] [CrossRef]
- Mahanta, S.K.; Suman, S.; Ghadei, S.K.; Balaji, U.; Sakthivel, R.; Sankaran, K.J. Direct Fabrication of Metal-Free Graphene Nanohairs/Polyimide Heterojunction for the Highly Efficient Photocatalytic Degradation of Industrial Dyes. Diam. Relat. Mater. 2023, 137, 110096. [Google Scholar] [CrossRef]
- Menon, D.M.N.; Giardino, M.; Janner, D. Direct Fabrication of Ultrahydrophobic Laser-Induced Graphene for Strain Sensors. Appl. Sci. 2023, 13, 4935. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Li, J.; Qu, M.; Kang, M.; Zhang, C. Nonmodified Laser-Induced Graphene Sensors for Lead-Ion Detection. ACS Appl. Nano Mater. 2023, 6, 3599–3607. [Google Scholar] [CrossRef]
- Samantaray, S.S.; Sangeetha, V.; Abinaya, S.; Ramaprabhu, S. Enhanced Hydrogen Storage Performance in Pd3Co Decorated Nitrogen/Boron Doped Graphene Composites. Int. J. Hydrogen Energy 2018, 43, 8018–8025. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Dong, X.; Mei, X.; Zhai, Z.; Zheng, B.; Lv, J.; Duan, W.; Wang, W. Formation of Hierarchical Porous Graphene Films with Defects Using a Nanosecond Laser on Polyimide Sheet. Appl. Surf. Sci. 2017, 419, 893–900. [Google Scholar] [CrossRef]
- Wang, F.; Dong, X.; Wang, K.; Duan, W.; Gao, M.; Zhai, Z.; Zhu, C.; Wang, W. Laser-Induced Nitrogen-Doped Hierarchically Porous Graphene for Advanced Electrochemical Energy Storage. Carbon 2019, 150, 396–407. [Google Scholar] [CrossRef]
- Mannan, M.A.; Hirano, Y.; Quitain, A.T.; Koinuma, M.; Kida, T. Graphene Oxide to B, N Co-Doped Graphene through Tris-Dimethylaminoborane Complex by Hydrothermal Implantation. Am. J. Mater. Sci. 2019, 2019, 22–28. [Google Scholar]
- Matsoso, B.J.; Ranganathan, K.; Mutuma, B.K.; Lerotholi, T.; Jones, G.; Coville, N.J. Time-Dependent Evolution of the Nitrogen Configurations in N-Doped Graphene Films. RSC Adv. 2016, 6, 106914–106920. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Xing, C.; Hu, L.; Wang, L.; Ding, M.; Zhang, H. High Energy Density Supercapacitor Based on N/B Co-Doped Graphene Nanoarchitectures and Ionic Liquid Electrolyte. Ionics 2019, 25, 4351–4360. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Tian, X.; Luo, L.; Fang, J.; Gao, H.; Jiang, Z.J. Hydrothermal Synthesis of Boron and Nitrogen Codoped Hollow Graphene Microspheres with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 19398–19407. [Google Scholar] [CrossRef]
- Molina-García, M.A.; Rees, N.V. “Metal-Free” Electrocatalysis: Quaternary-Doped Graphene and the Alkaline Oxygen Reduction Reaction. Appl. Catal. A Gen. 2018, 553, 107–116. [Google Scholar] [CrossRef]
- Chen, S.; Li, P.; Xu, S.; Pan, X.; Fu, Q.; Bao, X. Carbon Doping of Hexagonal Boron Nitride Porous Materials toward CO2 Capture. J. Mater. Chem. A Mater. 2018, 6, 1832–1839. [Google Scholar] [CrossRef]
- Zhou, S.; Zang, J.; Li, W.; Tian, P.; Gao, H.; Song, S.; Tian, X.; Wang, Y. B, N Co-Doped Nanocarbon Derived In Situ from Nanoboron Carbide as Electrocatalyst for Oxygen Reduction Reaction. ChemNanoMat 2021, 7, 200–206. [Google Scholar] [CrossRef]
- Coros, M.; Varodi, C.; Pogacean, F.; Gal, E.; Pruneanu, S.M. Nitrogen-Doped Graphene: The Influence of Doping Level on the Charge-Transfer Resistance and Apparent Heterogeneous Electron Transfer Rate. Sensors 2020, 20, 1815. [Google Scholar] [CrossRef]
- Bai, L.; Ge, Y.; Bai, L. Boron and Nitrogen Co-Doped Porous Carbons Synthesized from Polybenzoxazines for High-Performance Supercapacitors. Coatings 2019, 9, 657. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Hong, W.; Sun, J.; Wang, Z.; Ma, L.; Wang, J.; Yang, S. Pyrolytic Synthesis of Boron-Doped Graphene and Its Application Aselectrode Material for Supercapacitors. Electrochim. Acta 2013, 108, 666–673. [Google Scholar] [CrossRef]
- Sahoo, M.; Sreena, K.P.; Vinayan, B.P.; Ramaprabhu, S. Green Synthesis of Boron Doped Graphene and Its Application as High Performance Anode Material in Li Ion Battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Shao, C.; Qiu, S.; Chu, H.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Nitrogen-Doped Porous Microsphere Carbons Derived from Glucose and Aminourea for High-Performance Supercapacitors. Catal. Today 2018, 318, 150–156. [Google Scholar] [CrossRef]
- Gopalsamy, K.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Fabrication of Nitrogen and Sulfur Co-Doped Graphene Nanoribbons with Porous Architecture for High-Performance Supercapacitors. Chem. Eng. J. 2017, 312, 180–190. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, B.; Zhang, X.; Xie, X.; Jin, L.; Cao, Q. Synthesis of N-Doped Carbon Nanosheets with Controllable Porosity Derived from Bio-Oil for High-Performance Supercapacitors. J. Mater. Chem. A Mater. 2018, 6, 19653–19663. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Hanif, M.; Wang, Z.; Dong, L.; Dong, M. A Novel MnO2/Ti3C2Tx MXene Nanocomposite as High Performance Electrode Materials for Flexible Supercapacitors. Electrochim. Acta 2018, 290, 695–703. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.; Yang, X.; Long, G.; Zong, Y.; Li, X.; Zheng, X. Effect of Graphene Thickness on the Morphology Evolution of Hierarchical NiCoO2 Architectures and Their Superior Supercapacitance Performance. Ceram. Int. 2018, 44, 4875–4882. [Google Scholar] [CrossRef]
- Zhu, T.; Li, S.; Ren, B.; Zhang, L.; Dong, L.; Tan, L. Plasma-Induced Synthesis of Boron and Nitrogen Co-Doped Reduced Graphene Oxide for Super-Capacitors. J. Mater. Sci. 2019, 54, 9632–96420. [Google Scholar] [CrossRef]
- Huang, R.; Lv, J.; Chen, J.; Zhu, Y.; Zhu, J.; Wågberg, T.; Hu, G. Three-Dimensional Porous High Boron-Nitrogen-Doped Carbon for the Ultrasensitive Electrochemical Detection of Trace Heavy Metals in Food Samples. J. Hazard. Mater. 2023, 442, 130020. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 1119334063. [Google Scholar]
- Lin, X.; Lu, Z.; Zhang, Y.; Liu, B.; Mo, G.; Li, J.; Ye, J. A Glassy Carbon Electrode Modified with a Bismuth Film and Laser Etched Graphene for Simultaneous Voltammetric Sensing of Cd(II) and Pb(II). Microchim. Acta 2018, 185, 438. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A Sensitive Electrochemical Sensor Using an Iron Oxide/Graphene Composite for the Simultaneous Detection of Heavy Metal Ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P.P. Heteroatom-Doped Graphene as Sensing Materials: A Mini Review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef] [PubMed]
- Varodi, C.; Pogăcean, F.; Cioriță, A.; Pană, O.; Leoștean, C.; Cozar, B.; Radu, T.; Coroș, M.; Ștefan-Van Staden, R.I.; Pruneanu, S.M. Nitrogen and Sulfur Co-Doped Graphene as Efficient Electrode Material for l-Cysteine Detection. Chemosensors 2021, 9, 146. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, E.; Zhao, X. Glassy Carbon Electrode Modified by Conductive Polyaniline Coating for Determination of Trace Lead and Cadmium Ions in Acetate Buffer Solution. Thin Solid Film. 2011, 519, 5285–5289. [Google Scholar] [CrossRef]
- Sosa, V.; Barceló, C.; Serrano, N.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Antimony Film Screen-Printed Carbon Electrode for Stripping Analysis of Cd(II), Pb(II), and Cu(II) in Natural Samples. Anal. Chim. Acta 2015, 855, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Philips, M.F.; Gopalan, A.I.; Lee, K.P. Development of a Novel Cyano Group Containing Electrochemically Deposited Polymer Film for Ultrasensitive Simultaneous Detection of Trace Level Cadmium and Lead. J. Hazard. Mater. 2012, 237–238, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ruengpirasiri, P.; Punrat, E.; Chailapakul, O.; Chuanuwatanakul, S. Graphene Oxide-Modified Electrode Coated with in-Situ Antimony Film for the Simultaneous Determination of Heavy Metals by Sequential Injection-Anodic Stripping Voltammetry. Electroanalysis 2017, 29, 1022–1030. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Yang, B.; Xu, Q.; Xu, Q.; Hu, X. Electrochemical Sensor Construction Based on Nafion/Calcium Lignosulphonate Functionalized Porous Graphene Nanocomposite and Its Application for Simultaneous Detection of Trace Pb2+ and Cd2+. Sens. Actuators B Chem. 2018, 259, 540–551. [Google Scholar] [CrossRef]
- Varodi, C.; Pogăcean, F.; Coros, M.; Magerusan, L.; Stefan-Van Staden, R.I.; Pruneanu, S. Hydrothermal Synthesis of Nitrogen, Boron Co-doped Graphene with Enhanced Electro-catalytic Activity for Cymoxanil Detection. Sensors 2021, 21, 6630. [Google Scholar] [CrossRef] [PubMed]






| Samples | ID | IG | I2D | ID/IG | I2D/IG | LD | nD (×1011) | FWHM of 2D | La |
|---|---|---|---|---|---|---|---|---|---|
| LIGU | 1289.808 | 3848.702 | 2882.18 | 0.33 | 0.75 | 23.95 | 1.00 | 66.34 | 149.20 |
| LIGB | 2432.031 | 4749.7998 | 3623.687 | 0.51 | 0.76 | 19.37 | 1.53 | 70.70 | 97.65 |
| LIGN | 2970.914 | 4483.142 | 3272.021 | 0.66 | 0.73 | 17.03 | 1.99 | 80.12 | 75.45 |
| LIGBN | 6281.095 | 6513.148 | 4475.658 | 0.96 | 0.69 | 14.12 | 2.89 | 89.56 | 51.84 |
| Electrode | Electrochemical Techniques | Linear Range (µM) | LOD (µM) | Ref. | ||
|---|---|---|---|---|---|---|
| Pb2+ | Cd2+ | Pb2+ | Cd2+ | |||
| aPANI/bGCE | kSWASV | 0–2.0 | 0–2.0 | 0.10 | 0.13 | [59] |
| In situ cSbdSPCE | lDPASV | 0.08–0.30 | 0.10–0.60 | 20 | 30 | [60] |
| P(eDPA-co-2fABN)/GCE | mDPV | 1.25–283.2 | 11.20–8076 | 0.7963 | 2.2684 | [61] |
| SbgF/hGO/SPCE | nSI-SWASV | 0.1–1.3 | 0.3–1.5 | 0.026 | 0.054 | [62] |
| Nafion/iCLS/jPGR/GCE | DPASV | 0.05–5.0 | 0.05–5.0 | 0.01 | 0.003 | [63] |
| LIGBN | oSWV | 8–80 | 8–80 | 0.21 | 0.25 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, Y.; Suman, S.; Rakesh, B.; Ojha, G.P.; Deshpande, U.; Pant, B.; Sankaran, K.J. Boron and Nitrogen Co-Doped Porous Graphene Nanostructures for the Electrochemical Detection of Poisonous Heavy Metal Ions. Nanomaterials 2024, 14, 806. https://doi.org/10.3390/nano14090806
Chaudhary Y, Suman S, Rakesh B, Ojha GP, Deshpande U, Pant B, Sankaran KJ. Boron and Nitrogen Co-Doped Porous Graphene Nanostructures for the Electrochemical Detection of Poisonous Heavy Metal Ions. Nanomaterials. 2024; 14(9):806. https://doi.org/10.3390/nano14090806
Chicago/Turabian StyleChaudhary, Yogesh, Shradha Suman, Benadict Rakesh, Gunendra Prasad Ojha, Uday Deshpande, Bishweshwar Pant, and Kamatchi Jothiramalingam Sankaran. 2024. "Boron and Nitrogen Co-Doped Porous Graphene Nanostructures for the Electrochemical Detection of Poisonous Heavy Metal Ions" Nanomaterials 14, no. 9: 806. https://doi.org/10.3390/nano14090806







