Effectiveness of Systemic Insecticide Dog Treatment for the Control of Chagas Disease in the Tropics
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human, Dogs, and Other Transmitters (Rodents)
2.2. Dog Treatment
2.3. Treatment Strategies
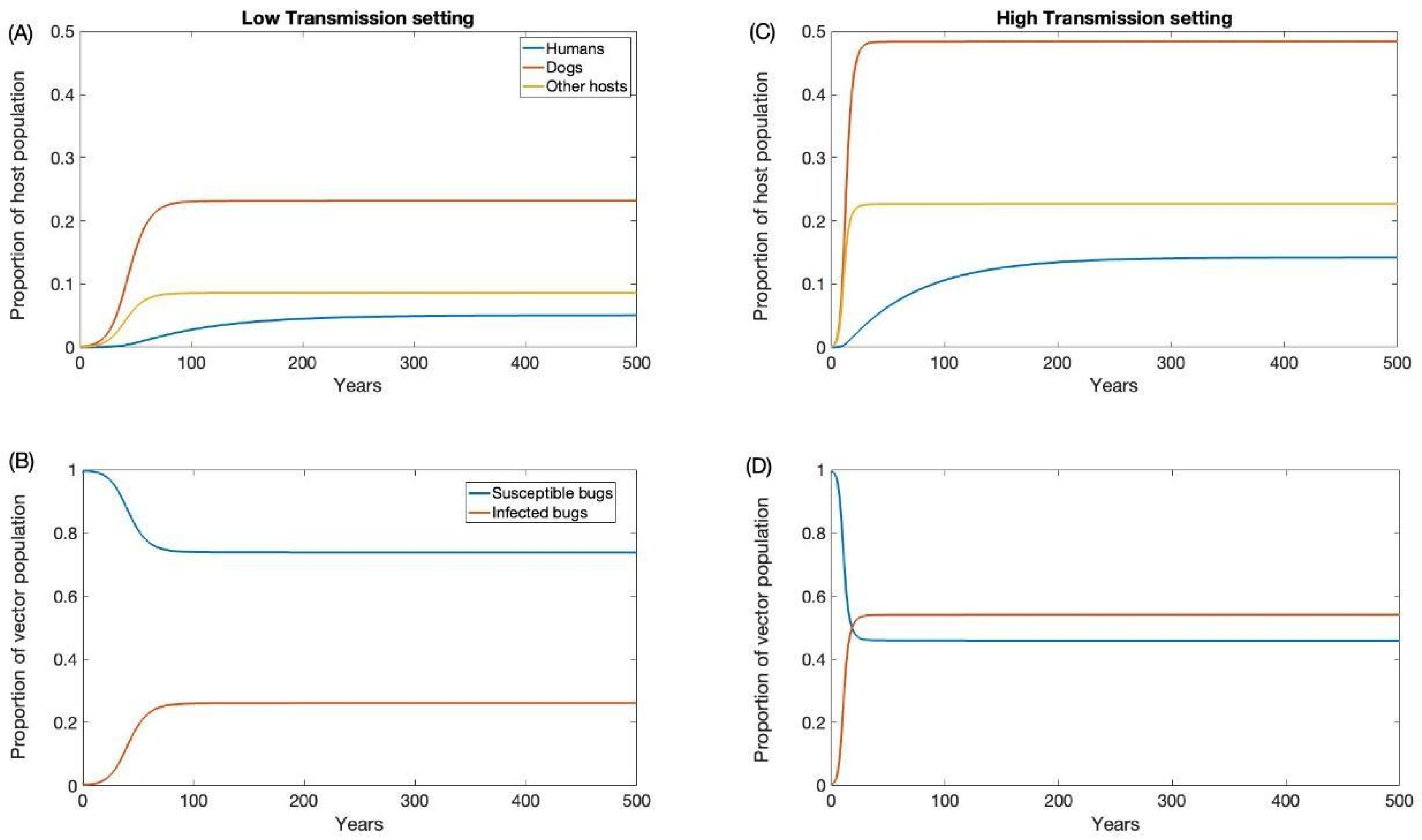
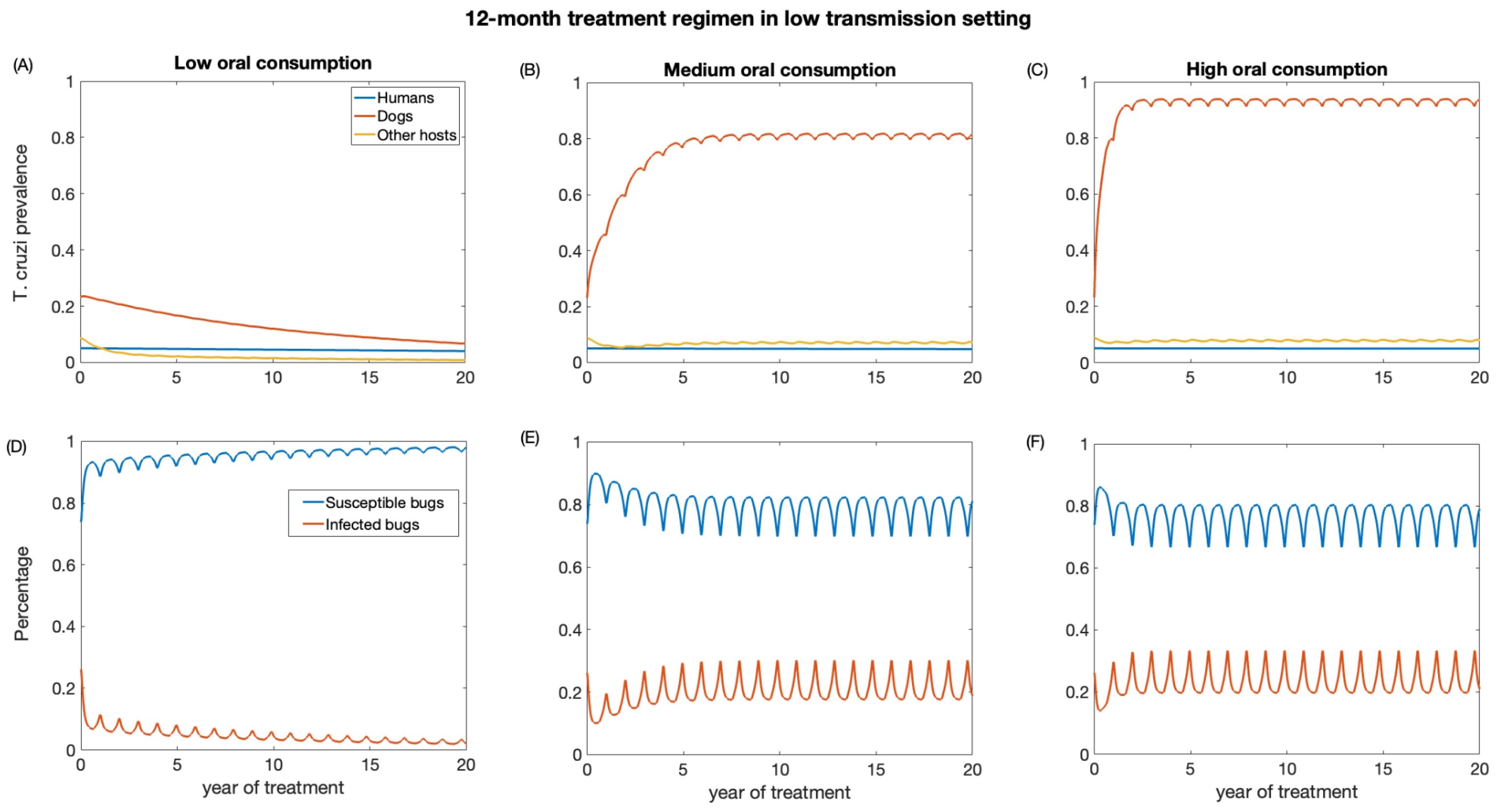
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tibayrenc, M.; Telleria, J. American Trypanosomiasis: Chagas Disease One Hundred Years of Research; Elsevier: Amsterdam, The Netherlands, 2010; Available online: https://books.google.com/books/about/American_Trypanosomiasis_Chagas_Disease.html?hl=&id=WQKeDAEACAAJ (accessed on 1 July 2023).
- Kribs-Zaleta, C. Estimating contact process saturation in sylvatic transmission of Trypanosoma cruzi in the United States. PLoS Negl. Trop. Dis. 2010, 4, e656. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.H.; Dorn, P.L.; Logan, J.L.; Stevens, L.; Pinnas, J.L.; Schmidt, J.O.; Klotz, S.A. “Kissing bugs”: Potential disease vectors and cause of anaphylaxis. Clin. Infect. Dis. 2010, 50, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Xavier, S.C.d.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Schmunis, G.A.; Yadon, Z.E. Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Arnal, A.; Waleckx, E.; Rico-Chávez, O.; Herrera, C.; Dumonteil, E. Estimating the current burden of Chagas disease in Mexico: A systematic review and meta-analysis of epidemiological surveys from 2006 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0006859. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Rojas, L.Z.; Echeverría, L.E.; Muka, T.; Franco, O.H. Global, Regional, and National Trends of Chagas Disease from 1990 to 2019: Comprehensive Analysis of the Global Burden of Disease Study. Glob Heart 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Blue Marble Health: An Innovative Plan to Fight Diseases of the Poor Amid Wealth; JHU Press: Baltimore, MD, USA, 2016; Available online: https://books.google.com/books/about/Blue_Marble_Health.html?hl=&id=thXUDAAAQBAJ (accessed on 1 July 2023).
- Lee, B.Y.; Bacon, K.M.; Bottazzi, M.E.; Hotez, P.J. Global economic burden of Chagas disease: A computational simulation model. Lancet Infect. Dis. 2013, 13, 342–348. [Google Scholar] [CrossRef]
- Gil-Santana, H.R.; Martins, D.d.S.; da Silva, J.B.; de Oliveira, J. First report of Microtriatoma borbai Lent & Wygodzinsky, 1979 (Hemiptera, Reduviidae, Triatominae) in the state of Espírito Santo, Brazil: Would M. borbai be living in eucalyptus crops? Rev. Soc. Bras. Med. Trop. 2021, 54, e0147-2021. [Google Scholar] [CrossRef]
- Chagas Disease (American Trypanosomiasis)—Fact Sheet (Revised in August 2012). Wkly. Epidemiol. Rec. 2012, 87, 519–522. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23311009 (accessed on 1 July 2023).
- Gürtler, R.E.; Kitron, U.; Cecere, M.C.; Segura, E.L.; Cohen, J.E. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc. Natl. Acad. Sci. USA 2007, 104, 16194–16199. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Disease-(American-Trypanosomiasis)-Epidemiology-and-Transmission. Available online: https://www.who.int/news-room/fact-sheets/detail (accessed on 3 July 2023).
- Curtis-Robles, R.; Meyers, A.C.; Auckland, L.D.; Zecca, I.B.; Skiles, R.; Hamer, S.A. Parasitic interactions among Trypanosoma cruzi, triatomine vectors, domestic animals, and wildlife in Big Bend National Park along the Texas-Mexico border. Acta Trop. 2018, 188, 225–233. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Committee on the Control of Chagas Disease, World Health Organization. Control of Chagas Disease: Second Report of the WHO Expert Committee; World Health Organization: Geneva, Switzerland, 2002; Available online: https://books.google.com/books/about/Control_of_Chagas_Disease.html?hl=&id=TaYsDwAAQBAJ (accessed on 3 July 2023).
- Roque, A.L.R.; Xavier, S.C.C.; da Rocha, M.G.; Duarte, A.C.M.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18981516 (accessed on 3 July 2023). [CrossRef] [PubMed]
- Finkelman, J. Innovative community-based ecosystem management for dengue and Chagas disease prevention in low and middle income countries in Latin America and the Caribbean. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 89–90. [Google Scholar] [CrossRef]
- Carcavallo, R.U. Atlas Dos Vetores Da Doença de Chagas Nas Américas. 1998. Available online: https://books.google.com/books/about/Atlas_Dos_Vetores_Da_Doen%C3%A7a_de_Chagas_N.html?hl=&id=ohRgAAAAMAAJ (accessed on 3 July 2023).
- Travi, B.L. Considering Dogs as Complementary Targets of Chagas Disease Control. Vector Borne Zoonotic Dis. 2019, 19, 90–94. [Google Scholar] [CrossRef]
- Cecere, M.C.; Gürtler, R.E.; Canale, D.; Chuit, R.; Cohen, J.E. The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev. Panam. Salud Publica 1997, 1, 273–279. [Google Scholar] [CrossRef]
- Macpherson, C.N.L.; Meslin, F.-X.; Wandeler, A.I. Dogs, Zoonoses and Public Health; CABI: Wallingford, UK, 2013; Available online: https://books.google.com/books/about/Dogs_Zoonoses_and_Public_Health.html?hl=&id=6F0n57jWPioC (accessed on 3 July 2023).
- Guarneri, A.; Lorenzo, M. Triatominae—The Biology of Chagas Disease Vectors; Springer Nature: Berlin/Heidelberg, Germany, 2021; Available online: https://play.google.com/store/books/details?id=Zz83EAAAQBAJ (accessed on 3 July 2023).
- Gürtler, R.E.; Cardinal, M.V. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015, 151, 32–50. [Google Scholar] [CrossRef]
- Ordóñez-Krasnowski, P.C.; Lanati, L.A.; Gaspe, M.S.; Cardinal, M.V.; Ceballos, L.A.; Gürtler, R.E. Domestic host availability modifies human-triatomine contact and host shifts of the Chagas disease vector Triatoma infestans in the humid Argentine Chaco. Med. Vet. Entomol. 2020, 34, 459–469. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Cecere, M.C.; Vázquez-Prokopec, G.M.; Ceballos, L.A.; Gurevitz, J.M.; Fernández, M.d.P.; Kitron, U.; Cohen, J.E. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl. Trop. Dis. 2014, 8, e2894. [Google Scholar] [CrossRef]
- Gurtler, R.E.; Canale, D.; Lauricella, M.A.; Cecere, M.C.; Castanera, M.B.; Segura, E.L.; Chuit, R.; Cohen, J.E. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am. J. Trop. Med. Hyg. 1996, 55, 24–31. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8702018 (accessed on 3 July 2023). [CrossRef]
- Gürtler, R.E.; Cecere, M.C.; Lauricella, M.A.; Cardinal, M.V.; Kitron, U.; Cohen, J.E. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology 2007, 134, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.A.; Castañera, M.B.; Gürtler, R.E.; Segura, E.L. Immunodiagnosis of Trypanosoma cruzi (Chagas’ disease) infection in naturally infected dogs. Mem. Inst. Oswaldo Cruz. 1998, 93, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Laiño, M.A.; Cardinal, M.V.; Enriquez, G.F.; Alvedro, A.; Gaspe, M.S.; Gürtler, R.E. An oral dose of Fluralaner administered to dogs kills pyrethroid-resistant and susceptible Chagas disease vectors for at least four months. Vet. Parasitol. 2019, 268, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Gürtler, R.E. Modeling household transmission of American trypanosomiasis. Science 2001, 293, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Loza, A.; Talaga, A.; Herbas, G.; Canaviri, R.J.; Cahuasiri, T.; Luck, L.; Guibarra, A.; Goncalves, R.; Pereira, J.A.; Gomez, S.A.; et al. Systemic insecticide treatment of the canine reservoir of Trypanosoma cruzi induces high levels of lethality in Triatoma infestans, a principal vector of Chagas disease. Parasit. Vectors 2017, 10, 344. [Google Scholar] [CrossRef]
- Reithinger, R.; Ceballos, L.; Stariolo, R.; Davies, C.R.; Gürtler, R.E. Chagas disease control: Deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 502–508. [Google Scholar] [CrossRef]
- Queiroga, T.B.D.; Gomez, L.C.P.; de Sena, E.R.; dos Santos, W.V.; Ferreira, H.R.P.; de Araújo-Neto, V.T.; Barbosa-Silva, A.N.; Brito, C.R.D.N.; Lima, R.K.d.R.; Fagundes-Neto, J.C.; et al. Insecticidal efficacy of fluralaner (Bravecto) against Triatoma brasiliensis, a major vector of Trypanosoma cruzi in Brazil. Parasit. Vectors. 2021, 14, 456. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Lauricella, M.A.; Cecere, M.C.; Cohen, J.E.; Segura, E.L.; Chuit, R.; Petersen, R.M. Incidence of trypanosoma cruzi infection among children following domestic reinfestation after insecticide spraying in rural northwestern Argentina. Am. J. Trop. Med. Hyg. 2005, 73, 95–103. Available online: https://www.ncbi.nlm.nih.gov/pubmed/16014842 (accessed on 5 July 2023). [CrossRef]
- Rokhsar, J.L.; Raynor, B.; Sheen, J.; Goldstein, N.D.; Levy, M.Z.; Castillo-Neyra, R. Modeling the impact of xenointoxication in dogs to halt Trypanosoma cruzi transmission. PLoS Comput. Biol. 2023, 19, e1011115. [Google Scholar] [CrossRef]
- Smith, D.L.; Battle, K.E.; Hay, S.I.; Barker, C.M.; Scott, T.W.; McKenzie, F.E. Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef]
- Reiner, R.C., Jr.; Perkins, T.A.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; Bisanzio, D.; et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J. R. Soc. Interface 2013, 10, 20120921. [Google Scholar] [CrossRef] [PubMed]
- Nouvellet, P.; Cucunubá, Z.M.; Gourbière, S. Ecology, evolution and control of Chagas disease: A century of neglected modelling and a promising future. Adv. Parasitol. 2015, 87, 135–191. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, M.C.; Schweigmann, N.J.; Bartoloni, N.J. Modelling inter-human transmission dynamics of Chagas disease: Analysis and application. Parasitology 2014, 141, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Steindorf, V.; Maidana, N.A. Modeling the Spatial Spread of Chagas Disease. Bull. Math. Biol. 2019, 81, 1687–1730. [Google Scholar] [CrossRef]
- Cucunubá, Z.M.; Nouvellet, P.; Peterson, J.K.; Bartsch, S.M.; Lee, B.Y.; Dobson, A.P.; Basáñez, M.-G. Complementary Paths to Chagas Disease Elimination: The Impact of Combining Vector Control with Etiological Treatment. Clin. Infect. Dis. 2018, 66, S293–S300. [Google Scholar] [CrossRef]
- Bartsch, S.M.; Peterson, J.K.; Hertenstein, D.L.; Skrip, L.; Ndeffo-Mbah, M.; Galvani, A.P.; Dobson, A.P.; Lee, B.Y. Comparison and validation of two computational models of Chagas disease: A thirty year perspective from Venezuela. Epidemics 2017, 18, 81–91. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Population Biology of Infectious Diseases: Report of the Dahlem Workshop on Population Biology of Infectious Disease Agents, Berlin 1982, March 14–19; Springer: Berlin/Heidelberg, Germany, 1982; Available online: https://books.google.com/books/about/Population_Biology_of_Infectious_Disease.html?hl=&id=MZ5rAAAAMAAJ (accessed on 11 July 2023).
- Ortega-Pacheco, A.; Rodriguez-Buenfil, J.C.; Bolio-Gonzalez, M.E.; Sauri-Arceo, C.H.; Jiménez-Coello, M.; Forsberg, C.L. A Survey of Dog Populations in Urban and Rural Areas of Yucatan, Mexico. Anthrozoös 2007, 20, 261–274. [Google Scholar] [CrossRef]
- Catala, S.S.; Gorla, D.E.; Basombrio, M.A. Vectorial transmission of Trypanosoma cruzi: An experimental field study with susceptible and immunized hosts. Am. J. Trop. Med. Hyg. 1992, 47, 20–26. [Google Scholar] [CrossRef]
- Levy, M.Z.; Tustin, A.; Castillo-Neyra, R.; Mabud, T.S.; Levy, K.; Barbu, C.M.; Quispe-Machaca, V.R.; Ancca-Juarez, J.; Borrini-Mayori, K.; Naquira-Velarde, C.; et al. Bottlenecks in domestic animal populations can facilitate the emergence of Trypanosoma cruzi, the aetiological agent of Chagas disease. Proc. Biol. Sci. 2015, 282, 20142807. [Google Scholar] [CrossRef]
- Lee, B.Y.; Bartsch, S.M.; Skrip, L.; Hertenstein, D.L.; Avelis, C.M.; Ndeffo-Mbah, M.; Tilchin, C.; Dumonteil, E.O.; Galvani, A. Are the London Declaration’s 2020 goals sufficient to control Chagas disease? Modeling scenarios for the Yucatan Peninsula. PLoS Negl. Trop. Dis. 2018, 12, e0006337. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Leal, J.A.; de Piñero, D.F. Domiciliary biting frequency and blood ingestion of the Chagas’s disease vector Rhodnius prolixus Ståhl (Hemiptera: Reduviidae), in Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, J.M.; Rendon, L.M.; Gonzalez, C.; Guhl, F. Using the basic reproduction number to assess the effects of climate change in the risk of Chagas disease transmission in Colombia. Acta Trop. 2013, 129, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lopez, J.A.; Jaramillio-Arango, C.J.; Martinez-Maya, J.J.; Alvarez Peralta, E.; Terrones, C.R. Study of the population structure of dogs in a political district in Mexico City. J. Anim. Vet. Adv. 2008, 7, 1352–1357. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/515774 (accessed on 11 July 2023).
- Cruz-Pacheco, G.; Esteva, L.; Vargas, C. Control measures for Chagas disease. Math. Biosci. 2012, 237, 49–60. [Google Scholar] [CrossRef]
- Arévalo, A.; Carranza, J.C.; Guhl, F.; Clavijo, J.A.; Vallejo, G.A. Comparison of the life cycles of Rhodnius colombiensis Moreno, Jurberg & Galvão, 1999 and R. prolixus Stal, 1872 (Hemiptera, Reduviidae, Triatominae) under laboratory conditions. Biomedica 2007, 27 (Suppl. S1), 119–129. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18154252 (accessed on 13 July 2023).
- Jiménez-Coello, M.; Guzmán-Marín, E.; Ortega-Pacheco, A.; Acosta-Viana, K.Y. Serological survey of American trypanosomiasis in dogs and their owners from an urban area of Mérida Yucatàn, México. Transbound. Emerg. Dis. 2010, 57, 33–36. [Google Scholar] [CrossRef]
- Estrada-Franco, J.G.; Bhatia, V.; Diaz-Albiter, H.; Ochoa-Garcia, L.; Barbabosa, A.; Vazquez-Chagoyan, J.C.; Martinez-Perez, M.A.; Guzman-Bracho, C.; Garg, N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 2006, 12, 624–630. [Google Scholar] [CrossRef]
- Jimenez-Coello, M.; Poot-Cob, M.; Ortega-Pacheco, A.; Guzman-Marin, E.; Ramos-Ligonio, A.; Sauri-Arceo, C.H.; Acosta-Viana, K.Y.; Bárcenas-Irabién, A.; López-Cancino, S.A.; Tun-Ku, E.; et al. American trypanosomiasis in dogs from an urban and rural area of Yucatan, Mexico. Vector Borne Zoonotic Dis. 2008, 8, 755–761. [Google Scholar] [CrossRef]
- Gürtler, R.E.; Ceballos, L.A.; Ordóñez-Krasnowski, P.; Lanati, L.A.; Stariolo, R.; Kitron, U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: Implications for the epidemiology of Chagas disease. PLoS Negl. Trop. Dis. 2009, 3, e447. [Google Scholar] [CrossRef]
- Coronado, X.; Rozas, M.; Botto-Mahan, C.; Ortíz, S.; Cattan, P.E.; Solari, A. Molecular epidemiology of Chagas disease in the wild transmission cycle: The evaluation in the sylvatic vector Mepraia spinolai from an endemic area of Chile. Am. J. Trop. Med. Hyg. 2009, 81, 656–659. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Poot-Ramos, A.; Chan-Pérez, J.I.; Gutiérrez-Blanco, E.; Acevedo-Arcique, C.M.; Baak-Baak, C.M.; Jiménez-Coello, M. Evaluation of the effectiveness of fluralaner against adult stages of Rhodnius prolixus in dogs. Parasitol. Int. 2022, 87, 102508. [Google Scholar] [CrossRef] [PubMed]
- Fiatsonu, E.; Busselman, R.E.; Hamer, G.L.; Hamer, S.A.; Ndeffo-Mbah, M.L. Effectiveness of fluralaner treatment regimens for the control of canine Chagas disease: A mathematical modeling study. PLoS Negl. Trop. Dis. 2023, 17, e0011084. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Description | Values | Reference |
|---|---|---|---|
| The transmission rate of T. cruzi from triatomine to other hosts | H: 0.000087/day L: 0.000058/day | Estimated from [46,47] | |
| The transmission rate of T. cruzi from triatomine to humans | H: 0.0000012/day L: 8 × 10−8/day | Estimated from [47,48,49] | |
| The transmission rate of T. cruzi from triatomine to dogs | H: 0.000025/day L: 0.000017/day | Estimated from [47,49,50] | |
| The transmission rate of T. cruzi from dogs to triatomine | H: 0.0086/day L: 0.0057/day | Estimated from [27,47,49] | |
| The transmission rate of T. cruzi from humans to triatomine | H: 0.000173/day L: 0.00011/day | Estimated from [47,49,50] | |
| The transmission rate of T. cruzi from other hosts to triatomine | H: 0.00754/day L: 0.005/day | [47,49,50] | |
| Human death rate | 0.00003641/day | [16] | |
| Dog death rate | 0.000455675/day | [51] | |
| Other competent death rate | 0.0027/day | [2] | |
| Triatomine death rate | 0.005/day | [52] | |
| κ | Carrying capacity of vectors per host | Estimated | From this study |
| The birth rate at carrying capacity | 0.09 | [53] | |
| ε | Transmission efficiency from infectious triatomine to susceptible dog via oral transmission | 0.1 | [2] |
| Triatomine population density | 31,600 vec/km2 | [2] | |
| Probability of triatomine infection per feed on dogs | 0.3082 | [27] | |
| α | Triatomine bites rate | 0.1428/day | [47] |
| Transmission Settings | Treatment Frequency | |||||
|---|---|---|---|---|---|---|
| Every Three Months | Every Twelve Months | |||||
| Dead Triatomine Consumption | Dead Triatomine Consumption | |||||
| Low | Medium | High | Low | Medium | High | |
| High | 80.40% | 61.70% | 58.90% | 74.00% | 52.60% | 49.30% |
| Low | 81.90% | 37.80% | 26.30% | 76.80% | 25.50% | 11.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiatsonu, E.; Deka, A.; Ndeffo-Mbah, M.L. Effectiveness of Systemic Insecticide Dog Treatment for the Control of Chagas Disease in the Tropics. Biology 2023, 12, 1235. https://doi.org/10.3390/biology12091235
Fiatsonu E, Deka A, Ndeffo-Mbah ML. Effectiveness of Systemic Insecticide Dog Treatment for the Control of Chagas Disease in the Tropics. Biology. 2023; 12(9):1235. https://doi.org/10.3390/biology12091235
Chicago/Turabian StyleFiatsonu, Edem, Aniruddha Deka, and Martial L. Ndeffo-Mbah. 2023. "Effectiveness of Systemic Insecticide Dog Treatment for the Control of Chagas Disease in the Tropics" Biology 12, no. 9: 1235. https://doi.org/10.3390/biology12091235








