BODIPY-Based Molecules for Biomedical Applications
Abstract
:1. Introduction
2. Properties of BODIPY-Based Compounds
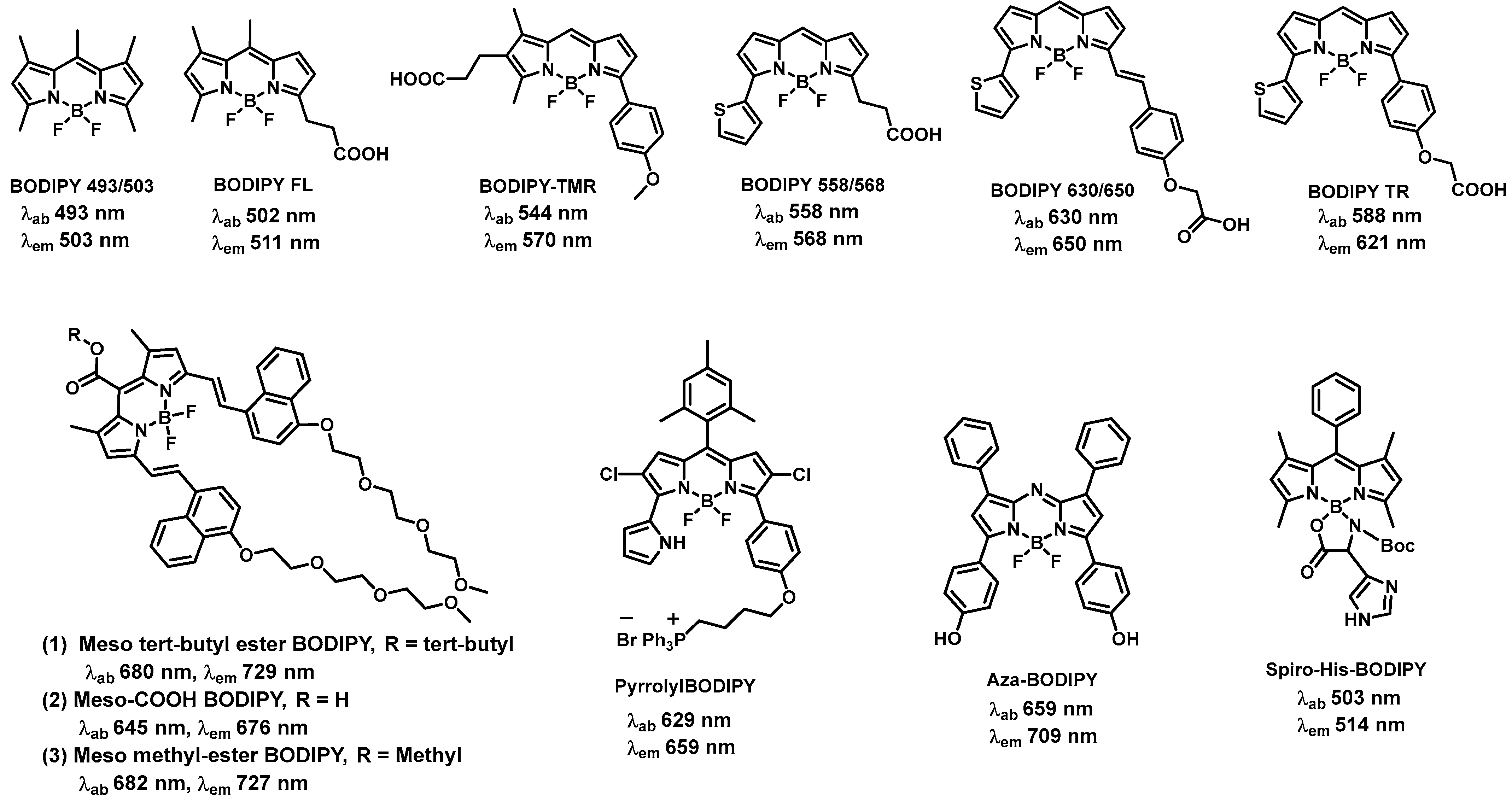
2.1. BODIPY-Based Fluorescent Compounds
2.2. BODIPY Probes Capable of Detecting and Tracking Amyloid-β Aggregates and Structural Changes
BODIPY Probes for Monitoring Aggregation and Conformational Changes of Amyloids
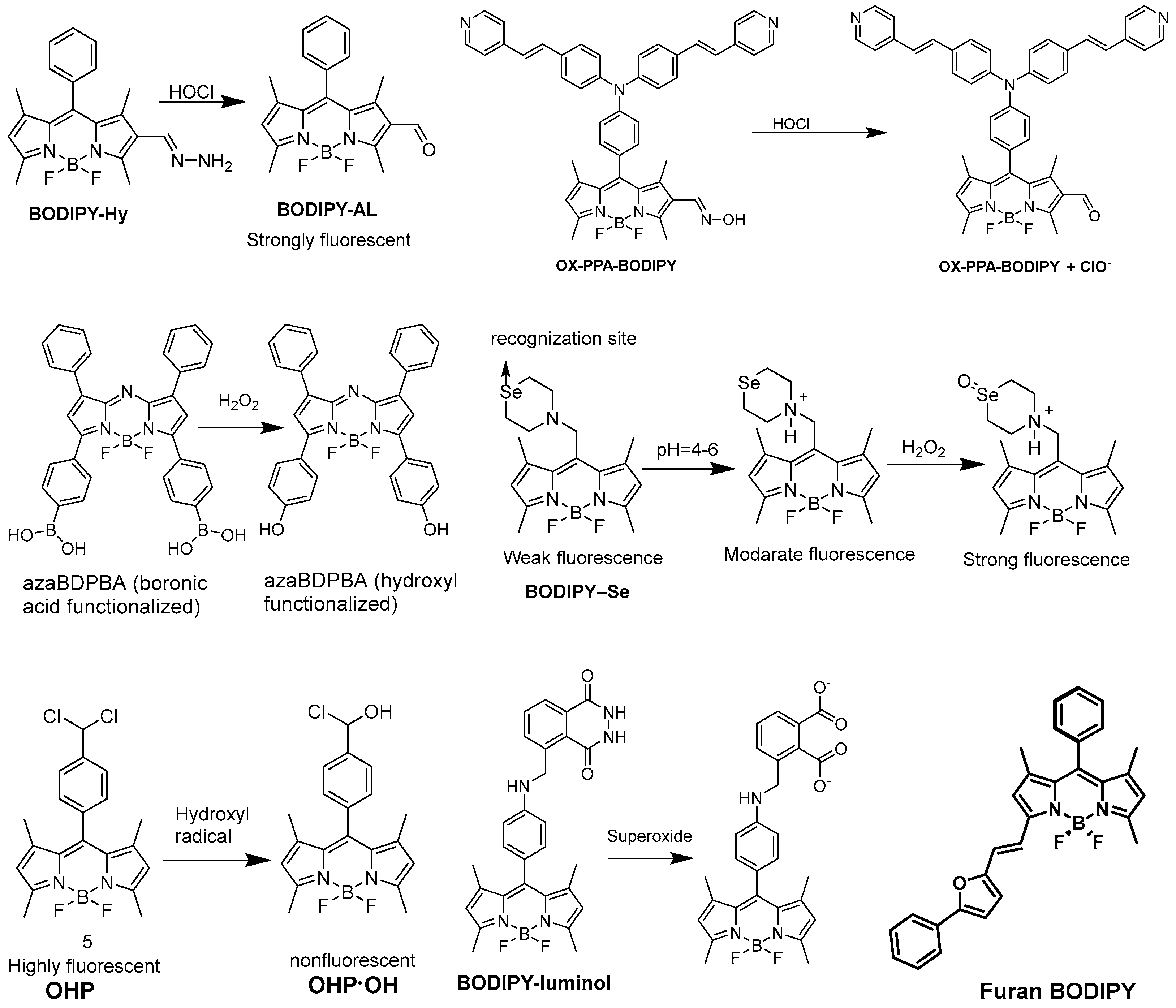
2.3. Detection of Reactive Oxygen Species
2.4. BODIPY-Based Probes in Cancer Detection
2.5. BODIPY Derivatives for PDT and PTT Applications
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Treibs, A.; Kreuzer, F.H. Difluorboryl—komplexe von di—und tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Cai, F.; Xu, X.; Fu, B.; Wang, S.; Shen, Z.; Tian, J.; Xu, H. BODIPY derivatives bearing borneol moieties: Enhancing cell membrane permeability for living cell imaging. Dye. Pigment. 2019, 164, 105–111. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Site—selective synthesis and characterization of BODIPY—acetylene copolymers and their transistor properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1978–1986. [Google Scholar] [CrossRef]
- Bandi, V.; Das, S.K.; Awuah, S.G.; You, Y.; D’Souza, F. Thieno-pyrrole-fused 4, 4-Difluoro-4-bora-3a, 4a-diaza-s-indacene–Fullerene dyads: Utilization of near-infrared sensitizers for ultrafast charge separation in donor–acceptor systems. J. Am. Chem. Soc. 2014, 136, 7571–7574. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Masilamani, G.; Agrawal, A.; Kumar, N.R.; Kumar, C.; Zade, S.S.; Bedi, A. Cyclopenta [c] thiophene-and diketopyrrolopyrrole-based red-green-blue electrochromic polymers. Org. Mater. 2022, 4, 268–276. [Google Scholar] [CrossRef]
- Gabe, Y.; Ueno, T.; Urano, Y.; Kojima, H.; Nagano, T. Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: Improvement of highly sensitive fluorescence probe for nitric oxide. Anal. Bioanal. Chem. 2006, 386, 621–626. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Bodipy dyes with tunable redox potentials and functional groups for further tethering: Preparation, electrochemical, and spectroscopic characterization. J. Am. Chem. Soc. 2010, 132, 17560–17569. [Google Scholar] [CrossRef]
- Marte, B. Cell division and cancer. Nature 2004, 432, 293–294. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-targeting imaging and theranostic agents—Current status and future perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhou, N.; Wu, C.-Y.; Li, S.; Chen, Y.-A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D. Validation of SV2A-targeted PET imaging for noninvasive assessment of neuroendocrine differentiation in prostate cancer. Int. J. Mol. Sci. 2021, 22, 13085. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [PubMed]
- Gonzalez, P.; Debnath, S.; Chen, Y.-A.; Hernandez, E.; Jha, P.; Dakanali, M.; Hsieh, J.-T.; Sun, X. A Theranostic Small-Molecule Prodrug Conjugate for Neuroendocrine Prostate Cancer. Pharmaceutics 2023, 15, 481. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Synthesis, optoelectronic, and transistor properties of BODIPY-and cyclopenta [c] thiophene-containing π-conjugated copolymers. J. Phys. Chem. C 2015, 119, 15859–15867. [Google Scholar] [CrossRef]
- Chapran, M.; Angioni, E.; Findlay, N.J.; Breig, B.; Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J.V.; Nastishin, Y.A. An ambipolar BODIPY derivative for a white exciplex OLED and cholesteric liquid crystal laser toward multifunctional devices. ACS Appl. Mater. Interfaces 2017, 9, 4750–4757. [Google Scholar] [CrossRef]
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY—based semiconducting materials for organic bulk heterojunction photovoltaics and thin—film transistors. ChemPlusChem 2019, 84, 18–37. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Huangfu, C.; Zhu, M.; Li, C.; Feng, L. The BODIPY-based chemosensor for the fluorometric determination of organochlorine pesticide dicofol. Food Chem. 2022, 370, 131033. [Google Scholar] [CrossRef]
- Li, F.-Z.; Wu, Z.; Lin, C.; Wang, Q.; Kuang, G.-C. Photophysical properties regulation and applications of BODIPY-based derivatives with electron donor-acceptor system. Results Chem. 2022, 4, 100384. [Google Scholar] [CrossRef]
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent progress in the development of fluorescent probes for detection of biothiols. Dye. Pigment. 2020, 177, 108321. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, B.; Liu, Y.; Hu, D.; Sheng, Z.; Zhang, X.; Yuan, Z. Molecular engineering of near-infrared light-responsive BODIPY-based nanoparticles with enhanced photothermal and photoacoustic efficiencies for cancer theranostics. Theranostics 2019, 9, 5315. [Google Scholar] [CrossRef]
- Li, M.; Tian, R.; Fan, J.; Du, J.; Long, S.; Peng, X. A lysosome-targeted BODIPY as potential NIR photosensitizer for photodynamic therapy. Dye. Pigment. 2017, 147, 99–105. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018, 358, 108–124. [Google Scholar] [CrossRef]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic small-molecule prodrug conjugates for targeted delivery and controlled release of toll-like receptor 7 agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]
- Amendoeira, A.F.; Luz, A.; Valente, R.; Roma-Rodrigues, C.; Ali, H.; van Lier, J.E.; Marques, F.; Baptista, P.V.; Fernandes, A.R. Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. Int. J. Mol. Sci. 2023, 24, 3600. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef]
- Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Piskorz, J.; Bocian, S.; Ziegler-Borowska, M.; Kędziera, D.; Kaczmarek-Kędziera, A. Photochemical properties and stability of BODIPY dyes. Int. J. Mol. Sci. 2021, 22, 6735. [Google Scholar] [CrossRef]
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-based molecules, a platform for photonic and solar cells. Molecules 2020, 26, 153. [Google Scholar] [CrossRef]
- Kumar, N.R.; Agrawal, A.R.; Debnath, S.; Choudhury, A.; Zade, S.S. Effect of connectivity variation in azulene-BODIPY triads and their optoelectronic properties. New J. Chem. 2023, 47, 2456–2463. [Google Scholar] [CrossRef]
- Duan, X.; Li, P.; Li, P.; Xie, T.; Yu, F.; Tang, B. The synthesis of polarity-sensitive fluorescent dyes based on the BODIPY chromophore. Dye. Pigment. 2011, 89, 217–222. [Google Scholar] [CrossRef]
- Glavaš, M.; Zlatić, K.; Jadreško, D.; Ljubić, I.; Basarić, N. Fluorescent pH sensors based on BODIPY structure sensitive in acidic media. Dye. Pigment. 2023, 220, 111660. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Brown, D.A. Fluorescent detection of lipid droplets and associated proteins. Curr. Protoc. Cell Biol. 2007, 35, 24.2.1–24.2.11. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Kanagawa, T.; Yamada, K.; Torimura, M.; Yokomaku, T.; Kamagata, Y.; Kurane, R. Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY® FL-labeled probe or primer. Nucleic Acids Res. 2001, 29, e34. [Google Scholar] [CrossRef] [PubMed]
- Guseva, G.B.; Antina, E.V.; Berezin, M.B.; Pavelyev, R.S.; Kayumov, A.R.; Ostolopovskaya, O.V.; Gilfanov, I.R.; Frolova, L.L.; Kutchin, A.V.; Akhverdiev, R.F. Design, spectral characteristics, and possibilities for practical application of BODIPY FL-labeled monoterpenoid. ACS Applied Bio. Mater. 2021, 4, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Clardy, S.M.; Mohan, J.F.; Vinegoni, C.; Keliher, E.J.; Iwamoto, Y.; Benoist, C.; Mathis, D.; Weissleder, R. Rapid, high efficiency isolation of pancreatic ß-cells. Sci. Rep. 2015, 5, 13681. [Google Scholar] [CrossRef]
- Brubaker, K.D.; Mao, F.; Gay, C.V. Localization of carbonic anhydrase in living osteoclasts with bodipy 558/568-modified acetazolamide, a thiadiazole carbonic anhydrase inhibitor. J. Histochem. Cytochem. 1999, 47, 545–550. [Google Scholar] [CrossRef]
- Herbert, S.M.; Leung, T.L.; Bishop, P.J. Fluorescent probes as a tool for labelling and tracking the amphibian chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2011, 96, 169–174. [Google Scholar] [CrossRef]
- Rae, J.; Fontaine, F.; Salim, A.A.; Lo, H.P.; Capon, R.J.; Parton, R.G.; Martin, S. High-throughput screening of Australian marine organism extracts for bioactive molecules affecting the cellular storage of neutral lipids. PLoS ONE 2011, 6, e22868. [Google Scholar] [CrossRef]
- Dale, C.L.; Hill, S.J.; Kellam, B. New potent, short-linker BODIPY-630/650™ labelled fluorescent adenosine receptor agonists. MedChemComm 2012, 3, 333–338. [Google Scholar] [CrossRef]
- Kok, Z.Y.; Stoddart, L.A.; Mistry, S.J.; Mocking, T.A.; Vischer, H.F.; Leurs, R.; Hill, S.J.; Mistry, S.N.; Kellam, B. Optimization of Peptide Linker-Based Fluorescent Ligands for the Histamine H1 Receptor. J. Med. Chem. 2022, 65, 8258–8288. [Google Scholar] [CrossRef]
- Cooper, M.S.; Szeto, D.P.; Sommers-Herivel, G.; Topczewski, J.; Solnica-Krezel, L.; Kang, H.C.; Johnson, I.; Kimelman, D. Visualizing morphogenesis in transgenic zebrafish embryos using BODIPY TR methyl ester dye as a vital counterstain for GFP. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 232, 359–368. [Google Scholar] [CrossRef]
- Ni, Y.; Zeng, L.; Kang, N.Y.; Huang, K.W.; Wang, L.; Zeng, Z.; Chang, Y.T.; Wu, J. meso—Ester and carboxylic acid substituted BODIPYs with far—red and near—infrared emission for bioimaging applications. Chem. A Eur. J. 2014, 20, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Guo, X.; Yan, X.; Shang, Y.; Yu, C.; Dai, E.; Jiang, T.; Hao, E.; Jiao, L. Red-to-Near-Infrared Emitting PyrrolylBODIPY Dyes: Synthesis, Photophysical Properties and Bioimaging Application. Chem. A Eur. J. 2023, 29, e202203832. [Google Scholar] [CrossRef] [PubMed]
- Pino, Y.C.; Aguilera, J.A.; Garcia-Gonzalez, V.; Alatorre-Meda, M.; Rodríguez-Velázquez, E.; Espinoza, K.A.; Frayde-Gomez, H.; Rivero, I.A. Synthesis of Aza-BODIPYs, Their Differential Binding for Cu (II), and Results of Bioimaging as Fluorescent Dyes of Langerhans β-Cells. ACS Omega 2022, 7, 42752–42762. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Bobadova-Parvanova, P.; Smith, K.M.; Vicente, M.G.H. Syntheses and Investigations of Conformationally Restricted, Linker-Free α-Amino Acid–BODIPYs via Boron Functionalization. J. Org. Chem. 2021, 86, 18030–18041. [Google Scholar] [CrossRef]
- Pavliukeviciene, B.; Zentelyte, A.; Jankunec, M.; Valiuliene, G.; Talaikis, M.; Navakauskiene, R.; Niaura, G.; Valincius, G. Amyloid β oligomers inhibit growth of human cancer cells. PLoS ONE 2019, 14, e0221563. [Google Scholar] [CrossRef]
- Jin, W.-S.; Bu, X.-L.; Liu, Y.-H.; Shen, L.-L.; Zhuang, Z.-Q.; Jiao, S.-S.; Zhu, C.; Wang, Q.-H.; Zhou, H.-D.; Zhang, T. Plasma amyloid-beta levels in patients with different types of cancer. Neurotox. Res. 2017, 31, 283–288. [Google Scholar] [CrossRef]
- Qin, H.; Cui, T.; Liu, Z.; Zhou, Y.; Niu, J.; Ren, J.; Qu, X. Engineering amyloid aggregation as a new way to eliminate cancer stem cells by the disruption of iron homeostasis. Nano Lett. 2021, 21, 7379–7387. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.W.; Alonso, A.; Brown, C.M.; Dzyuba, S.V. Triazole-containing BODIPY dyes as novel fluorescent probes for soluble oligomers of amyloid Aβ1–42 peptide. Biochem. Biophys. Res. Commun. 2010, 391, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, F.; Kolemen, S.; Kumada, H.-O.; Ono, M.; Saji, H.; Akkaya, E.U. Designing BODIPY-based probes for fluorescence imaging of β-amyloid plaques. RSC Adv. 2014, 4, 51032–51037. [Google Scholar] [CrossRef]
- Ono, M.; Ishikawa, M.; Kimura, H.; Hayashi, S.; Matsumura, K.; Watanabe, H.; Shimizu, Y.; Cheng, Y.; Cui, M.; Kawashima, H. Development of dual functional SPECT/fluorescent probes for imaging cerebral β-amyloid plaques. Bioorganic Med. Chem. Lett. 2010, 20, 3885–3888. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Watanabe, H.; Kimura, H.; Saji, H. BODIPY-based molecular probe for imaging of cerebral β-amyloid plaques. ACS Chem. Neurosci. 2012, 3, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sutharsan, J.; Dakanali, M.; Capule, C.C.; Haidekker, M.A.; Yang, J.; Theodorakis, E.A. Rational design of amyloid binding agents based on the molecular rotor motif. ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Ono, M.; Matsumura, K.; Yoshimura, M.; Kimura, H.; Saji, H. Molecular imaging of β-amyloid plaques with near-infrared boron dipyrromethane (BODIPY)-based fluorescent probes. Mol. Imaging 2013, 12, 7290.2013.00049. [Google Scholar] [CrossRef]
- Jameson, L.P.; Smith, N.W.; Dzyuba, S.V. Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (Aβ) self-assembly. ACS Chem. Neurosci. 2012, 3, 807–819. [Google Scholar] [CrossRef]
- Smith, N.W.; Annunziata, O.; Dzyuba, S.V. Amphotericin B interactions with soluble oligomers of amyloid Aβ1-42 peptide. Bioorganic Med. Chem. 2009, 17, 2366–2370. [Google Scholar] [CrossRef]
- Tonali, N.; Dodero, V.I.; Kaffy, J.; Hericks, L.; Ongeri, S.; Sewald, N. Real—Time BODIPY—Binding Assay To Screen Inhibitors of the Early Oligomerization Process of Aβ1-42 Peptide. ChemBioChem 2020, 21, 1129–1135. [Google Scholar] [CrossRef]
- Wang, E.; Qiao, H.; Zhou, Y.; Pang, L.; Yu, F.; Zhang, J.; Ma, T. A novel “turn-on” fluorogenic probe for sensing hypochlorous acid based on BODIPY. RSC Adv. 2015, 5, 73040–73045. [Google Scholar] [CrossRef]
- Xu, X.-X.; Qian, Y. A novel pyridyl triphenylamine–BODIPY aldoxime: Naked-eye visible and fluorometric chemodosimeter for hypochlorite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 356–361. [Google Scholar] [CrossRef]
- Xu, J.; Zhai, J.; Xu, Y.; Zhu, J.; Qin, Y.; Jiang, D. A near-infrared fluorescent aza-bodipy probe for dual-wavelength detection of hydrogen peroxide in living cells. Analyst 2016, 141, 2380–2383. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qian, Y. A selenamorpholine-based redox-responsive fluorescent probe for targeting lysosome and visualizing exogenous/endogenous hydrogen peroxide in living cells and zebrafish. J. Mater. Chem. B 2019, 7, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Zhu, M.; Yan, C.; Ma, Y.; Guo, Z.; Zhu, W. Rational design of ratiometric near-infrared aza-BODIPY-based fluorescent probe for in vivo imaging of endogenous hydrogen peroxide. ACS Appl. Bio Mater. 2019, 3, 45–52. [Google Scholar] [CrossRef]
- Lei, K.; Sun, M.; Du, L.; Zhang, X.; Yu, H.; Wang, S.; Hayat, T.; Alsaedi, A. Sensitive determination of endogenous hydroxyl radical in live cell by a BODIPY based fluorescent probe. Talanta 2017, 170, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Boyle, C.J.; Zhou, D.; Wong, B.M.; Kittilstved, K.R.; Venkataraman, D. Persistent radical anion polymers based on naphthalenediimide and a vinylene spacer. RSC Adv. 2018, 8, 14760–14764. [Google Scholar] [CrossRef]
- Bag, S.; Tseng, J.-C.; Rochford, J. A BODIPY-luminol chemiluminescent resonance energy-transfer (CRET) cassette for imaging of cellular superoxide. Org. Biomol. Chem. 2015, 13, 1763–1767. [Google Scholar] [CrossRef]
- Kaya, S.; Ismaiel, Y.A.; Kwon, N.; Kim, G.; Bila, J.L.; Yoon, J.; Seven, O.; Akkaya, E.U. Imaging of intracellular singlet oxygen with bright BODIPY dyes. Dye. Pigment. 2021, 188, 109158. [Google Scholar] [CrossRef]
- Zhang, P.-L.; Wang, Z.-K.; Chen, Q.-Y.; Du, X.; Gao, J. Biocompatible G-Quadruplex/BODIPY assembly for cancer cell imaging and the attenuation of mitochondria. Bioorganic Med. Chem. Lett. 2019, 29, 1943–1947. [Google Scholar] [CrossRef]
- Tiwari, R.; Shinde, P.S.; Sreedharan, S.; Dey, A.K.; Vallis, K.A.; Mhaske, S.B.; Pramanik, S.K.; Das, A. Photoactivatable prodrug for simultaneous release of mertansine and CO along with a BODIPY derivative as a luminescent marker in mitochondria: A proof of concept for NIR image-guided cancer therapy. Chem. Sci. 2021, 12, 2667–2673. [Google Scholar] [CrossRef]
- Xiong, H.; Kos, P.; Yan, Y.; Zhou, K.; Miller, J.B.; Elkassih, S.; Siegwart, D.J. Activatable water-soluble probes enhance tumor imaging by responding to dysregulated pH and exhibiting high tumor-to-liver fluorescence emission contrast. Bioconjugate Chem. 2016, 27, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Zhang, L.; Li, Y.; Zhu, D.; Xie, Z.; Li, G.; Zhang, P.; Su, Z.; Dou, C.; Tang, B.Z. Ultrafast and noninvasive long-term bioimaging with highly stable red aggregation-induced emission nanoparticles. Anal. Chem. 2019, 91, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Bedi, A.; Debnath, S.; Chandak, H.S.; Zade, S.S. Phenyl-capped cyclopenta [c] chalcogenophenes: Synthesis, crystal structures, electrochemistry and theoretical insights. RSC Adv. 2014, 4, 35653–35658. [Google Scholar] [CrossRef]
- Chen, N.; Kommidi, H.; Guo, H.; Wu, A.P.; Zhang, Z.; Yang, X.; Xia, L.; An, F.; Ting, R. A lysosome specific, acidic-pH activated, near-infrared Bodipy fluorescent probe for noninvasive, long-term, in vivo tumor imaging. Mater. Sci. Eng. C 2020, 111, 110762. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.-Y.; Fan, L.; Shen, H.; Wu, B.; Kong, S.; Luo, Y.; Huang, Z.-S.; Ye, X. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dye. Pigment. 2022, 197, 109897. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Zheng, M.; Xie, Z. Near-infrared BODIPY-paclitaxel conjugates assembling organic nanoparticles for chemotherapy and bioimaging. J. Colloid Interface Sci. 2018, 514, 584–591. [Google Scholar] [CrossRef]
- Kong, X.; Di, L.; Fan, Y.; Zhou, Z.; Feng, X.; Gai, L.; Tian, J.; Lu, H. Lysosome-targeting turn-on red/NIR BODIPY probes for imaging hypoxic cells. Chem. Commun. 2019, 55, 11567–11570. [Google Scholar] [CrossRef]
- Caruso, E.; Gariboldi, M.; Sangion, A.; Gramatica, P.; Banfi, S. Synthesis, photodynamic activity, and quantitative structure-activity relationship modelling of a series of BODIPYs. J. Photochem. Photobiol. B Biol. 2017, 167, 269–281. [Google Scholar] [CrossRef]
- Caruso, E.; Malacarne, M.C.; Marras, E.; Papa, E.; Bertato, L.; Banfi, S.; Gariboldi, M.B. New BODIPYs for photodynamic therapy (PDT): Synthesis and activity on human cancer cell lines. Bioorganic Med. Chem. 2020, 28, 115737. [Google Scholar] [CrossRef]
- Kim, B.; Sui, B.; Yue, X.; Tang, S.; Tichy, M.G.; Belfield, K.D. In Vitro Photodynamic Studies of a BODIPY—Based Photosensitizer. Eur. J. Org. Chem. 2017, 2017, 25–28. [Google Scholar] [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martinez, S.R.; Cosa, G. Reactive oxygen species mediated activation of a dormant singlet oxygen photosensitizer: From autocatalytic singlet oxygen amplification to chemicontrolled photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [Google Scholar] [CrossRef]
- Epelde-Elezcano, N.; Martínez-Martínez, V.; Pena-Cabrera, E.; Gómez-Durán, C.F.; Arbeloa, I.L.; Lacombe, S. Modulation of singlet oxygen generation in halogenated BODIPY dyes by substitution at their meso position: Towards a solvent-independent standard in the vis region. RSC Adv. 2016, 6, 41991–41998. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Ji, X.; Lin, G.; Xu, S.; Dong, X.; Zhao, W. Discovery of a monoiodo aza-BODIPY near-infrared photosensitizer: In vitro and in vivo evaluation for photodynamic therapy. J. Med. Chem. 2020, 63, 9950–9964. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bai, J.; Qian, Y. Synthesis of a triphenylamine BODIPY photosensitizer with D–A configuration and its application in intracellular simulated photodynamic therapy. New J. Chem. 2019, 43, 16829–16834. [Google Scholar] [CrossRef]
- Wang, C.; Qian, Y. A water soluble carbazolyl-BODIPY photosensitizer with an orthogonal D–A structure for photodynamic therapy in living cells and zebrafish. Biomater. Sci. 2020, 8, 830–836. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the intersystem crossing mechanism in a helical BODIPY for low--dose photodynamic therapy. Angew. Chem. 2020, 132, 16248–16255. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-atom-free photosensitizers: From molecular design to applications in the photodynamic therapy of cancer. Acc. Chem. Res. 2020, 54, 207–220. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-atom-free BODIPY photosensitizers with intersystem crossing mediated by intramolecular photoinduced electron transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Agarwal, A.; Kumar, N.R.; Bedi, A. Selenium-Based Drug Development for Antioxidant and Anticancer Activity. Future Pharmacol. 2022, 2, 595–607. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, T.-I.; Kim, H.; Choi, Y.; Kim, Y. Photoactivatable BODIPY platform: Light-triggered anticancer drug release and fluorescence monitoring. ACS Appl. Bio Mater. 2019, 2, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, S.; Deng, Z.; Tse, M.-K.; Matsuda, Y.; Zhu, G. BODI-Pt, a green-light-activatable and carboplatin-based platinum (iv) anticancer prodrug with enhanced activation and cytotoxicity. Inorg. Chem. 2020, 59, 11823–11833. [Google Scholar] [CrossRef]
- Sampedro, A.; Ramos-Torres, Á.; Schwöppe, C.; Mück-Lichtenfeld, C.; Helmers, I.; Bort, A.; Díaz-Laviada, I.; Fernández, G. Hierarchical self--assembly of BODIPY dyes as a tool to improve the antitumor activity of capsaicin in prostate cancer. Angew. Chem. Int. Ed. 2018, 57, 17235–17239. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Mao, Z.; Kim, J.H.; Lee, J.; Xiong, H.; Zhang, F.; Kim, J.S. Engineering of BODIPY-based theranostics for cancer therapy. Coord. Chem. Rev. 2023, 476, 214908. [Google Scholar] [CrossRef]
- Yang, K.; Zhao, S.; Li, B.; Wang, B.; Lan, M.; Song, X. Low temperature photothermal therapy: Advances and perspectives. Coord. Chem. Rev. 2022, 454, 214330. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza—BODIPY for imaging—guided synergistic photodynamic and photothermal tumor therapy. Adv. Healthc. Mater. 2018, 7, 1800606. [Google Scholar] [CrossRef]
- Zou, Y.; Long, S.; Xiong, T.; Zhao, X.; Sun, W.; Du, J.; Fan, J.; Peng, X. Single-molecule forster resonance energy transfer-based photosensitizer for synergistic photodynamic/photothermal therapy. ACS Cent. Sci. 2021, 7, 327–334. [Google Scholar] [CrossRef]
- Chen, K.; Dong, Y.; Zhao, X.; Imran, M.; Tang, G.; Zhao, J.; Liu, Q. Bodipy derivatives as triplet photosensitizers and the related intersystem crossing mechanisms. Front. Chem. 2019, 7, 821. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L.T.; Dogan, A.L.; Guc, D. Designing excited states: Theory—guided access to efficient photosensitizers for photodynamic action. Angew. Chem. Int. Ed. 2011, 50, 11937–11941. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cui, X.; Zhao, J. Hetero Bodipy-dimers as heavy atom-free triplet photosensitizers showing a long-lived triplet excited state for triplet–triplet annihilation upconversion. Chem. Commun. 2013, 49, 9009–9011. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Dey, S.; Patra, S.; Bera, A.; Ghosh, T.; Prasad, B.; Sayala, K.D.; Maji, K.; Bedi, A.; Debnath, S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules 2023, 13, 1723. https://doi.org/10.3390/biom13121723
Das S, Dey S, Patra S, Bera A, Ghosh T, Prasad B, Sayala KD, Maji K, Bedi A, Debnath S. BODIPY-Based Molecules for Biomedical Applications. Biomolecules. 2023; 13(12):1723. https://doi.org/10.3390/biom13121723
Chicago/Turabian StyleDas, Sarasija, Sudipto Dey, Sanujit Patra, Arindam Bera, Totan Ghosh, Bibin Prasad, Kapil Dev Sayala, Krishnendu Maji, Anjan Bedi, and Sashi Debnath. 2023. "BODIPY-Based Molecules for Biomedical Applications" Biomolecules 13, no. 12: 1723. https://doi.org/10.3390/biom13121723




