Gas Flow-Dependent Modification of Plasma Chemistry in μAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma/CAP Source
2.2. Determination of Evaporation and Temperature
2.3. Determination of pH Values
2.4. Measurement of Dissolved Oxygen
2.5. Detection of Nitrite and Nitrate
2.6. Measurement of H2O2
2.7. Measurement of Nitric Oxide and Nitrogen Dioxide
2.8. Cell Culture
2.9. Plasma Treatments of Fibroblasts
2.10. Toxicity, Viability and Proliferation
2.11. Statistical Analysis
3. Results
3.1. Plasma Characterization
3.2. Impact of CAP on Physicochemical Modifications of Aqueous Solutions
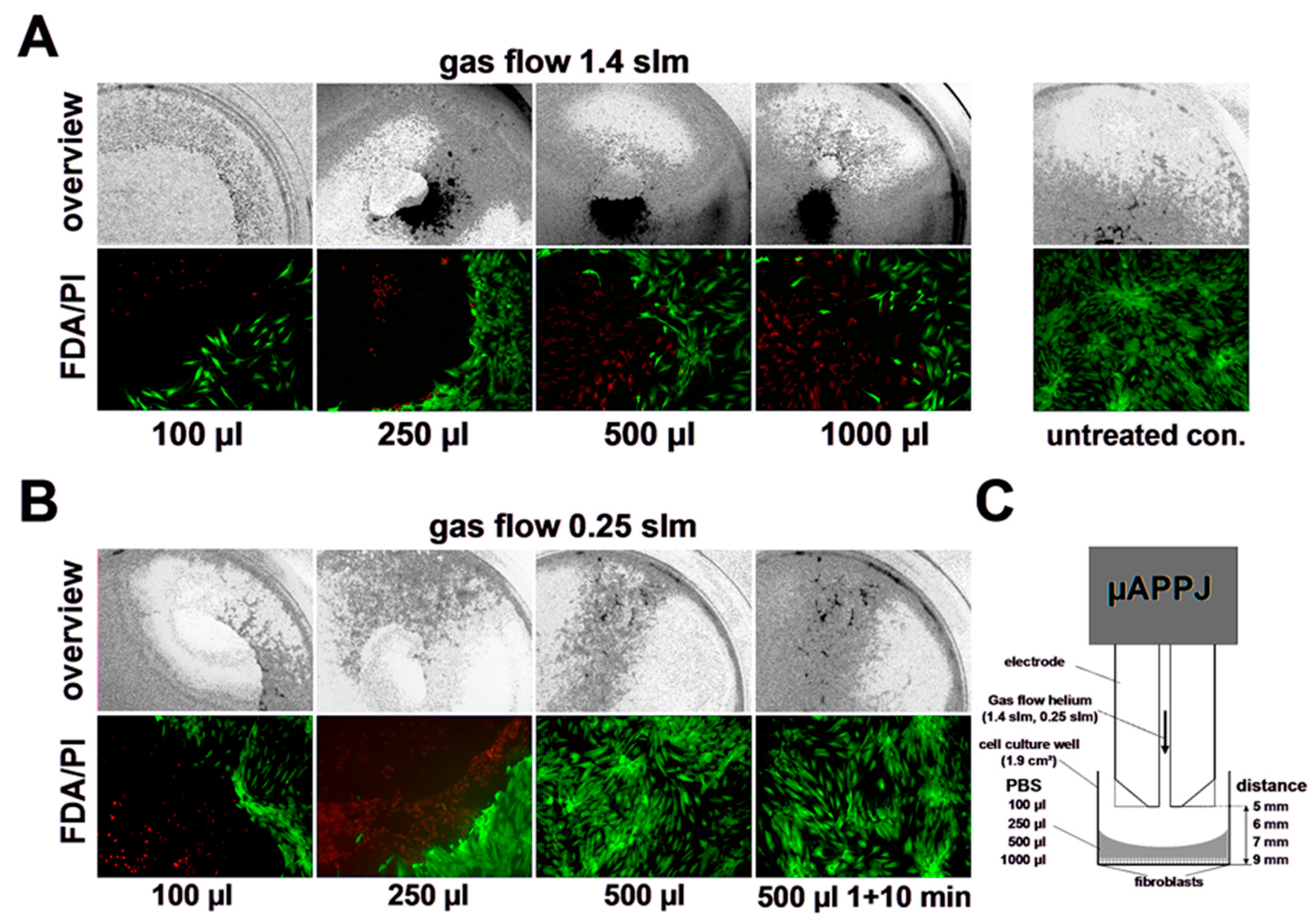
3.3. Impact of Helium Stream Exposure on Viability of Primary Human Skin Fibroblasts
3.4. Impact of CAP on Viability of Primary Human Skin Fibroblasts
3.5. Impact of CAP on Proliferation Capacity of Primary Human Skin Fibroblasts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, K.; Schoenbach, K.; Eden, J. Microplasmas and applications. J. Phys. D Appl. Phys. 2006, 39, R55. [Google Scholar] [CrossRef]
- Laroussi, M.; Akan, T. Arc-Free Atmospheric Pressure Cold Plasma Jets: A Review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; El-Habachi, A.; Shi, W.; Ciocca, M. High-pressure hollow cathode discharges. Plasma Sources Sci. Technol. 1997, 6, 468–477. [Google Scholar] [CrossRef]
- Eden, J.G.; Park, S.-J.; Ostrom, N.P.; McCain, S.T.; Wagner, C.J.; Vojak, B.A.; Chen, J.; Liu, C.; Von Allmen, P.; Zenhausern, F.; et al. Microplasma devices fabricated in silicon, ceramic, and metal/polymer structures: Arrays, emitters and photodetectors. J. Phys. D Appl. Phys. 2003, 36, 2869–2877. [Google Scholar] [CrossRef]
- Baars-Hibbe, L.; Sichler, P.; Schrader, C.; Lucas, N.; Gericke, K.-H.; Büttgenbach, S. High frequency glow discharges at atmospheric pressure with micro-structured electrode arrays. J. Phys. D Appl. Phys. 2005, 38, 510–517. [Google Scholar] [CrossRef]
- Kong, M.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Graves, D.B. Reactive Species from Cold Atmospheric Plasma: Implications for Cancer Therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Hemke, T.; Wollny, A.; Gebhardt, M.; Brinkmann, R.P.; Mussenbrock, T. Spatially resolved simulation of a radio-frequency driven micro-atmospheric pressure plasma jet and its effluent. J. Phys. D Appl. Phys. 2011, 44, 285206. [Google Scholar] [CrossRef]
- McKay, K.; Liu, D.X.; Rong, M.Z.; Iza, F.; Kong, M.G. Dynamics and particle fluxes in atmospheric-pressure electronegative radio frequency microplasmas. Appl. Phys. Lett. 2011, 99, 091501. [Google Scholar] [CrossRef]
- Niemi, K.; Reuter, S.; Graham, L.M.; Waskoenig, J.; Gans, T. Diagnostic based modeling for determining absolute atomic oxygen densities in atmospheric pressure helium-oxygen plasmas. Appl. Phys. Lett. 2009, 95, 151504. [Google Scholar] [CrossRef]
- Niemi, K.; Waskoenig, J.; Sadeghi, N.; Gans, T.; O’Connell, D. The role of helium metastable states in radio-frequency driven helium–oxygen atmospheric pressure plasma jets: Measurement and numerical simulation. Plasma Sources Sci. Technol. 2011, 20, 055005. [Google Scholar] [CrossRef]
- Shi, J.J.; Kong, M.G. Mechanisms of the α and γ modes in radio-frequency atmospheric glow discharges. J. Appl. Phys. 2005, 97, 023306. [Google Scholar] [CrossRef]
- Yang, A.; Wang, X.; Rong, M.; Liu, D.; Iza, F.; Kong, M.G. 1-D fluid model of atmospheric-pressure rf He+O2 cold plasmas: Parametric study and critical evaluation. Phys. Plasmas 2011, 18, 113503. [Google Scholar] [CrossRef]
- Ellerweg, D.; Benedikt, J.; von Keudell, A.; Knake, N.; Schulz-von der Gathen, V. Characterization of the effluent of a He/O2 microscale atmospheric pressure plasma jet by quantitative molecular beam mass spectrometry. New J. Phys. 2010, 12, 013021. [Google Scholar] [CrossRef]
- Herrmann, H.W.; Henins, I.; Park, J.; Selwyn, G.S. Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet (APPJ). Phys. Plasmas 1999, 6, 2284–2289. [Google Scholar] [CrossRef]
- Gibson, A.R.; McCarthy, H.O.; Ali, A.A.; O’Connell, D.; Graham, W.G. Interactions of a Non-Thermal Atmospheric Pressure Plasma Effluent with PC-3 Prostate Cancer Cells. Plasma Process. Polym. 2014, 11, 1142–1149. [Google Scholar] [CrossRef]
- O’connell, D.; Cox, L.J.; Hyland, W.B.; McMahon, S.J.; Reuter, S.; Graham, W.G.; Gans, T.; Currell, F.J. Cold atmospheric pressure plasma jet interactions with plasmid DNA. Appl. Phys. Lett. 2011, 98, 043701. [Google Scholar] [CrossRef]
- Coulombe, S.; Léveillé, V.; Yonson, S.; Leask, R.L. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl. Chem. 2006, 78, 1147–1156. [Google Scholar] [CrossRef]
- Foest, R.; Kindel, E.; Lange, H.; Ohl, A.; Stieber, M.; Weltmann, K.-D. RF Capillary Jet—A Tool for Localized Surface Treatment. Contrib. Plasma Phys. 2007, 47, 119–128. [Google Scholar] [CrossRef]
- Voráč, J.; Dvořák, P.; Procházka, V.; Ehlbeck, J.; Reuter, S. Measurement of hydroxyl radical (OH) concentration in an argon RF plasma jet by laser-induced fluorescence. Plasma Sources Sci. Technol. 2013, 22, 025016. [Google Scholar] [CrossRef]
- Robert, E.; Barbosa, E.; Dozias, S.; Vandamme, M.; Cachoncinlle, C.; Viladrosa, R.; Pouvesle, J.M. Experimental Study of a Compact Nanosecond Plasma Gun. Plasma Process. Polym. 2009, 6, 795–802. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; Von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44, 013002. [Google Scholar] [CrossRef]
- von Woedtke, T.; Metelmann, H.-R.; Weltmann, K.-D. Clinical Plasma Medicine: State and Perspectives of in Vivo Application of Cold Atmospheric Plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- der Gathen, V.S.-V.; Buck, V.; Gans, T.; Knake, N.; Niemi, K.; Reuter, S.; Schaper, L.; Winter, J. Optical Diagnostics of Micro Discharge Jets. Contrib. Plasma Phys. 2007, 47, 510–519. [Google Scholar] [CrossRef]
- der Gathen, V.S.-V.; Schaper, L.; Knake, N.; Reuter, S.; Niemi, K.; Gans, T.; Winter, J. Spatially resolved diagnostics on a microscale atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2008, 41, 194004. [Google Scholar] [CrossRef]
- Golda, J.; Kogelheide, F.; Awakowicz, P.; der Gathen, V.S.-V. Dissipated electrical power and electron density in an RF atmospheric pressure helium plasma jet. Plasma Sources Sci. Technol. 2019, 28, 095023. [Google Scholar] [CrossRef]
- Golda, J.; Held, J.; Redeker, B.; Konkowski, M.; Beijer, P.; Sobota, A.; Kroesen, G.; Braithwaite, N.S.J.; Reuter, S.; Turner, M.M.; et al. Concepts and characteristics of the ‘COST Reference Microplasma Jet’. J. Phys. D Appl. Phys. 2016, 49, 084003. [Google Scholar] [CrossRef]
- Kelly, S.; Golda, J.; Turner, M.M.; der Gathen, V.S.-V. Gas and heat dynamics of a micro-scaled atmospheric pressure plasma reference jet. J. Phys. D Appl. Phys. 2015, 48, 444002. [Google Scholar] [CrossRef]
- Preissing, P.; Korolov, I.; Schulze, J.; der Gathen, V.S.-V.; Böke, M. Three-dimensional density distributions of NO in the effluent of the COST reference microplasma jet operated in He/N2/O2. Plasma Sources Sci. Technol. 2020, 29, 125001. [Google Scholar] [CrossRef]
- Steuer, D.; Korolov, I.; Chur, S.; Schulze, J.; der Gathen, V.S.-V.; Golda, J.; Böke, M. 2D spatially resolved O atom density profiles in an atmospheric pressure plasma jet: From the active plasma volume to the effluent. J. Phys. D Appl. Phys. 2021, 54, 355204. [Google Scholar] [CrossRef]
- Frank, S.; Kämpfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric oxide drives skin repair: Novel functions of an established mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef]
- Suschek, C.V.; Schewe, T.; Sies, H.; Kroncke, K.D. Nitrite, a naturally occurring precursor of nitric oxide that acts like a ‘prodrug’. Biol. Chem. 2006, 387, 499–506. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bruch-Gerharz, D.; Schnorr, O.; Suschek, C.; Beck, K.-F.; Pfeilschifter, J.; Ruzicka, T.; Kolb-Bachofen, V. Arginase 1 Overexpression in Psoriasis: Limitation of inducible nitric oxide synthase activity as a molecular mechanism for keratinocyte hy-perproliferation. Am. J. Pathol. 2003, 162, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.; Pankotai, E.; Fehér, M.; Somlai, A.; Kiss, L.; Bíró, L.; Szabó, C.; Kollai, M.; de Oliveira, M.; Lacza, Z. S-nitrosoglutathione-containing hydrogel increases dermal blood flow in streptozotocin-induced diabetic rats. Br. J. Dermatol. 2007, 156, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Salyapongse, A.N.; Bragdon, G.A.; Shears, L.L.; Watkins, S.C.; Edington, H.D.; Billiar, T.R. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am. J. Physiol. 1999, 277, H1600–H1608. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Strandhoy, J.; White, W.L. Nitric oxide synthase activity is up-regulated in melanoma cell lines: A potential mechanism for metastases formation. Melanoma Res. 1996, 6, 121–126. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Kabisov, R.K.; Pekshev, A.V.; Kozlov, N.P.; Perov Iu, L. Experimental clinical substantiation of plasma dy-namic therapy of wounds with nitric oxide. Biulleten’ Eksperimental’noi Biol. I Meditsiny 1998, 126, 210–215. [Google Scholar]
- Shekhter, A.B.; Serezhenkov, V.A.; Rudenko, T.G.; Pekshev, A.V.; Vanin, A.F. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide 2005, 12, 210–219. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Liebmann, J.; Scherer, J.; Bibinov, N.; Rajasekaran, P.; Kovacs, R.; Gesche, R.; Awakowicz, P.; Kolb-Bachofen, V. Biological effects of nitric oxide generated by an atmospheric pressure gas-plasma on human skin cells. Nitric Oxide 2010, 24, 8–16. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Haertel, B.; Hähnel, M.; Blackert, S.; Wende, K.; von Woedtke, T.; Lindequist, U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biol. Int. 2012, 36, 1217–1222. [Google Scholar] [CrossRef]
- Haertel, B.; Straßenburg, S.; Oehmigen, K.; Wende, K.; von Woedtke, T.; Lindequist, U. Differential Influence of Components Resulting from Atmospheric-Pressure Plasma on Integrin Expression of Human HaCaT Keratinocytes. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blackert, S.; Haertel, B.; Wende, K.; von Woedtke, T.; Lindequist, U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (HaCaT). J. Dermatol. Sci. 2013, 70, 173–181. [Google Scholar] [CrossRef]
- Shi, X.-M.; Zhang, G.-J.; Yuan, Y.-K.; Ma, Y.; Xu, G.-M.; Yang, Y. Effects of Low-Temperature Atmospheric Air Plasmas on the Activity and Function of Human Lymphocytes. Plasma Process. Polym. 2008, 5, 482–488. [Google Scholar] [CrossRef]
- Haertel, B.; Volkmann, F.; von Woedtke, T.; Lindequist, U. Differential sensitivity of lymphocyte subpopulations to non-thermal atmospheric-pressure plasma. Immunobiology 2012, 217, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Barekzi, N.; Laroussi, M. Effects of Low Temperature Plasmas on Cancer Cells. Plasma Process. Polym. 2013, 10, 1039–1050. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Li, Y.-F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Shereshevsky, A.; Jost, M.M.; Brooks, A.D.; Fridman, A.; Gutsol, A.; Vasilets, V.; Friedman, G. Floating Electrode Dielectric Barrier Discharge Plasma in Air Promoting Apoptotic Behavior in Melanoma Skin Cancer Cell Lines. Plasma Chem. Plasma Process. 2007, 27, 163–176. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, J.-H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.-S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.-O. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens. Bioelectron. 2011, 28, 333–338. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, S.U.; Kim, K.I.; Kang, S.; Shin, Y.S.; Chang, J.W.; Yang, S.S.; Lee, K.; Lee, J.-S.; Moon, E.; et al. Nonthermal Plasma Induces Apoptosis in ATC Cells: Involvement of JNK and p38 MAPK-Dependent ROS. Yonsei Med. J. 2014, 55, 1640–1647. [Google Scholar] [CrossRef]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, Z.; Zou, F.; Zhao, S.; Lu, X.; Yang, G.; He, G.; Ostrikov, K.K. Plasma-Induced Death of HepG2 Cancer Cells: Intracellular Effects of Reactive Species. Plasma Process. Polym. 2012, 9, 59–66. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Bröker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free. Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef]
- Haertel, B.; Wende, K.; Von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Exp. Dermatol. 2011, 20, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K.P.; Friedman, G.; Fridman, A.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface 2012, 9, 147–157. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.; Kalghatgi, S.; Cerchar, E.; Fridman, G.; Shereshevsky, A.; Torabi, B.; Arjunan, K.P.; Podolsky, E.; Fridman, A.; Friedman, G.; et al. Non-thermal Plasma Induces Apoptosis in Melanoma Cells via Production of Intracellular Reactive Oxygen Species. Ann. Biomed. Eng. 2011, 39, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Chen, L.C.; Suzuki, H.; Mori, K.; Ariyada, O.; Hiraoka, K. Mass Spectrometric Detection of Gaseous Hydrogen Peroxide in Ambient Air Using Dielectric Barrier Discharge as an Excitation Source. Chem. Lett. 2009, 38, 520–521. [Google Scholar] [CrossRef]
- Pieraggi, M.T.; Bouissou, H.; Angelier, C.; Uhart, D.; Magnol, J.P.; Kokolo, J. The fibroblast. Ann. Pathol. 1985, 5, 65–76. [Google Scholar]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Feelisch, M.; Rassaf, T.; Mnaimneh, S.; Singh, N.; Bryan, N.S.; Jourd’Heuil, D.; Kelm, M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: Implications for the fate of NO in vivo. FASEB J. 2002, 16, 1775–1785. [Google Scholar] [CrossRef]
- Suschek, C.V.; Paunel, A.; Kolb-Bachofen, V. Nonenzymatic Nitric Oxide Formation during UVA Irradiation of Human Skin: Experimental Setups and Ways to Measure. Methods Enzymol. 2005, 396, 568–578. [Google Scholar] [CrossRef]
- Yang, F.; Troncy, E.; Francœur, M.; Vinet, B.; Vinay, P.; Czaika, G.; Blaise, G. Effects of reducing reagents and temperature on conversion of nitrite and nitrate to nitric oxide and detection of NO by chemiluminescence. Clin. Chem. 1997, 43, 657–662. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric determination of hydrogen peroxide using potassium titanium(IV) oxalate. Anal. 1980, 105, 950–954. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Heuer, K.; Demir, E.; Hoffmanns, M.A.; Baldus, S.; Fuchs, P.C.; Awakowicz, P.; Suschek, C.V.; Opländer, C. Non-Thermal Dielectric Barrier Discharge (DBD) Effects on Proliferation and Differentiation of Human Fibroblasts Are Primary Mediated by Hydrogen Peroxide. PLoS ONE 2015, 10, e0144968. [Google Scholar] [CrossRef] [PubMed]
- Hegmann, L.; Sturm, S.; Niegisch, G.; Windolf, J.; Suschek, C.V. Enhancement of human bladder carcinoma cell chemosensitivity to Mitomycin C through quasi-monochromatic blue light (λ = 453 ± 10 nm). J. Photochem. Photobiol. B Biol. 2022, 236, 112582. [Google Scholar] [CrossRef]
- Ermolaeva, S.A.; Sysolyatina, E.V.; Gintsburg, A.L. Atmospheric pressure nonthermal plasmas for bacterial biofilm prevention and eradication. Biointerphases 2015, 10, 029404. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, M.; Merbahi, N.; Pathak, A.; Eichwald, O. Low-temperature plasmas at atmospheric pressure: Toward new pharmaceutical treatments in medicine. Fundam. Clin. Pharmacol. 2014, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Nosenko, T.; Shimizu, T.; Steffes, B.; Zimmermann, J.; Stolz, W.; Schmidt, H.U.; Isbary, G.; Pompl, R.; Bunk, W.; Fujii, S.; et al. Low-Temperature Atmospheric-Pressure Plasmas as a Source of Reactive Oxygen and Nitrogen Species for Chronic Wound Disinfection. Free Radic. Bio. Med. 2009, 47, S128. [Google Scholar]
- Bruch-Gerharz, D.; Ruzicka, T.; Kolb-Bachofen, V. Nitric oxide and its implications in skin homeostasis and disease—a review. Arch. Dermatol. Res. 1998, 290, 643–651. [Google Scholar] [CrossRef]
- Cals-Grierson, M.-M.; Ormerod, A.D. Nitric oxide function in the skin. Nitric Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Kumar, A.; Arora, S. Understanding the Potential Role and Delivery Approaches of Nitric Oxide in Chronic Wound Healing Management. Curr. Pharm. Des. 2021, 27, 1999–2014. [Google Scholar] [CrossRef]
- Soneja, A.; Drews, M.; Malinski, T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol. Rep. 2005, 57, 108–119. [Google Scholar]
- Isenberg, J.S.; Ridnour, L.A.; Espey, M.G.; Wink, D.A.; Roberts, D.A. Nitric oxide in wound-healing. Microsurgery 2005, 25, 442–451. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Feibel, D.; Kwiatkowski, A.; Opländer, C.; Grieb, G.; Windolf, J.; Suschek, C.V. Enrichment of Bone Tissue with Antibacterially Effective Amounts of Nitric Oxide Derivatives by Treatment with Dielectric Barrier Discharge Plasmas Optimized for Nitrogen Oxide Chemistry. Biomedicines 2023, 11, 244. [Google Scholar] [CrossRef]
- Golda, J.; Sgonina, K.; Held, J.; Benedikt, J.; der Gathen, V.S.-V. Treating Surfaces with a Cold Atmospheric Pressure Plasma using the COST-Jet. J. Vis. Exp. 2020, 165, e61801. [Google Scholar] [CrossRef]
- Suschek, C.V.; Feibel, D.; von Kohout, M.; Opländer, C. Enhancement of Nitric Oxide Bioavailability by Modulation of Cutaneous Nitric Oxide Stores. Biomedicines 2022, 10, 2124. [Google Scholar] [CrossRef]
- de Bono, D.; Yang, W. Exposure to low concentrations of hydrogen peroxide causes delayed endothelial cell death and inhibits proliferation of surviving cells. Atherosclerosis 1995, 114, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ames, B.N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. USA 1994, 91, 4130–4134. [Google Scholar] [CrossRef] [PubMed]
- Mammone, T.; Ingrassia, M.; Goyarts, E. Osmotic stress induces terminal differentiation in cultured normal human epidermal keratinocytes. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Matsumoto, K.; Gon, Y.; Nakayama, T.; Takeshita, I.; Horie, T. Hyperosmolarity-induced Interleukin-8 Expression in Human Bronchial Epithelial Cells through p38 Mitogen-activated Protein Kinase. Am. J. Respir. Crit. Care Med. 1999, 159, 634–640. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feibel, D.; Golda, J.; Held, J.; Awakowicz, P.; Schulz-von der Gathen, V.; Suschek, C.V.; Opländer, C.; Jansen, F. Gas Flow-Dependent Modification of Plasma Chemistry in μAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts. Biomedicines 2023, 11, 1242. https://doi.org/10.3390/biomedicines11051242
Feibel D, Golda J, Held J, Awakowicz P, Schulz-von der Gathen V, Suschek CV, Opländer C, Jansen F. Gas Flow-Dependent Modification of Plasma Chemistry in μAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts. Biomedicines. 2023; 11(5):1242. https://doi.org/10.3390/biomedicines11051242
Chicago/Turabian StyleFeibel, Dennis, Judith Golda, Julian Held, Peter Awakowicz, Volker Schulz-von der Gathen, Christoph V. Suschek, Christian Opländer, and Florian Jansen. 2023. "Gas Flow-Dependent Modification of Plasma Chemistry in μAPP Jet-Generated Cold Atmospheric Plasma and Its Impact on Human Skin Fibroblasts" Biomedicines 11, no. 5: 1242. https://doi.org/10.3390/biomedicines11051242





