Drug-Induced Myopathies: A Comprehensive Review and Update
Abstract
1. Introduction
2. Objectives
3. Methods
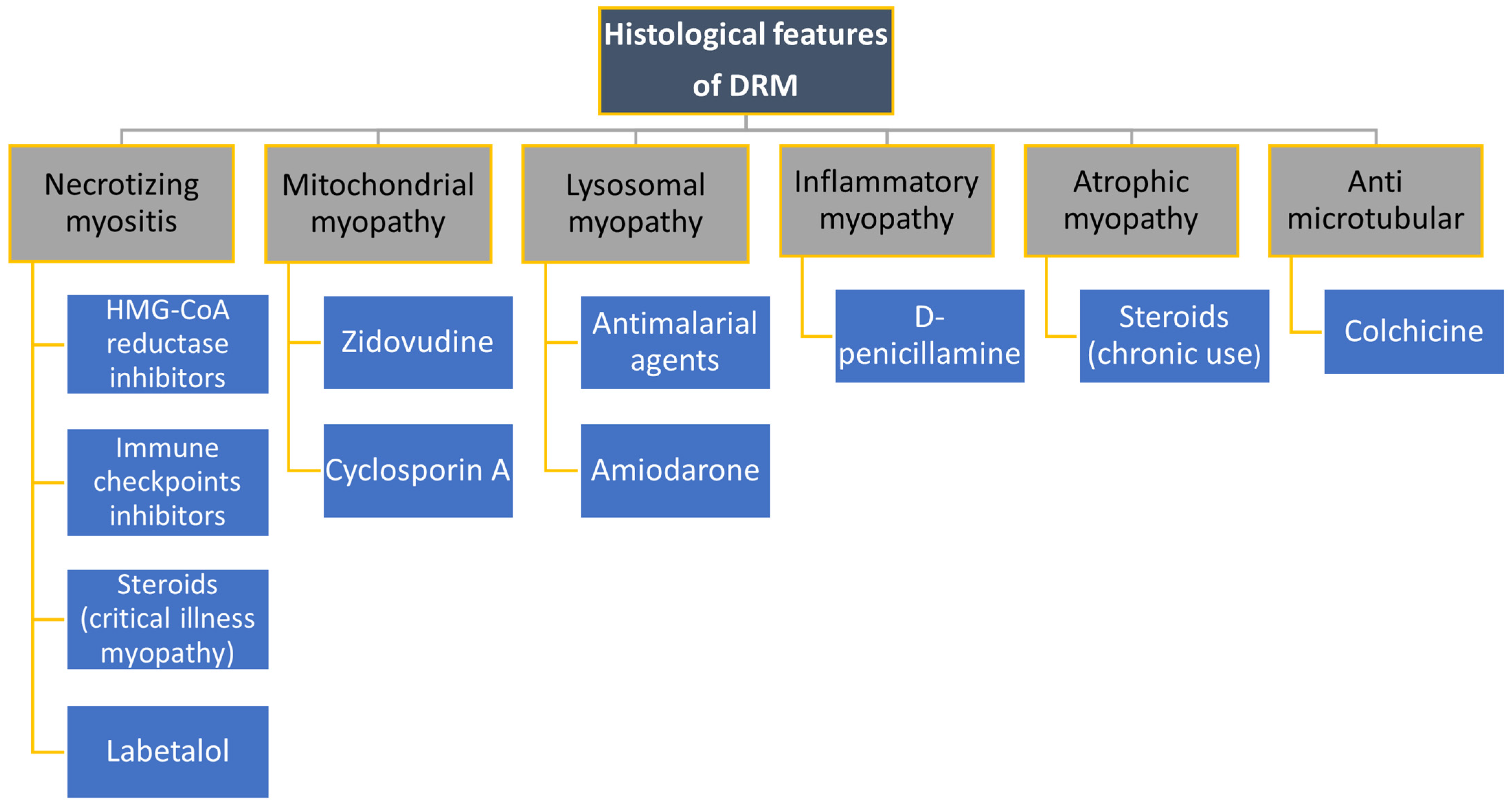
4. Drugs Causing Myopathies
4.1. Hypolipemic Medicines
4.2. Cardiological Drugs
4.3. Colchicine
4.4. Steroids
4.5. Antimalarials
4.6. Cyclosporine A
4.7. Zidovudine
4.8. D-Penicillamine
4.9. Checkpoint Inhibitors
5. Limitations of the Review
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cimmino, M.A.; Ferrone, C.; Cutolo, M. Epidemiology of chronic musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2011, 25, 173–183. [Google Scholar] [CrossRef]
- Alonso, R.; Cuevas, A.; Cafferata, A. Diagnosis and Management of Statin Intolerance. J. Atheroscler. Thromb. 2019, 26, 207–215. [Google Scholar] [CrossRef]
- Abed, W.; Abujbara, M.; Batieha, A.; Ajlouni, K. Statin Induced Myopathy Among Patients Attending the National Center for Diabetes, endocrinology, & genetics. Ann. Med. Surg. 2022, 74, 103304. [Google Scholar] [CrossRef] [PubMed]
- Ucar, M.; Mjörndal, T.; Dahlqvist, R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000, 22, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Phillips, C.O.; Foody, J.M.; Wang, Y.; Mangalmurti, S.; Ko, D.T.; Krumholz, H.M. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation 2006, 114, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Bégaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–The PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Koro, C.E. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin. Ther. 2007, 29, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Stępień, K.M.; Tomaszewska, J.; Czuczwar, S.J. Statin-induced myopathies. Pharmacol. Rep. 2011, 63, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Miller, K.; Bayliss, M.; Sanchez, R.J.; Baccara-Dinet, M.T.; Chibedi-De-Roche, D.; Taylor, B.; Khan, I.; Manvelian, G.; White, M.; et al. The Statin-Associated Muscle Symptom Clinical Index (SAMS-CI): Revision for Clinical Use, Content Validation, and Inter-rater Reliability. Cardiovasc. Drugs Ther. 2017, 31, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, R.C.; Smith, S.C., Jr.; Bairey-Merz, C.N.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke 2002, 33, 2337–2341. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef]
- Turner, R.M.; Pirmohamed, M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J. Clin. Med. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, A.; Neely, D.; Armitage, J.; Chinoy, H.; Cooper, R.G.; Laaksonen, R.; Carr, D.F.; Bloch, K.M.; Fahy, J.; Hanson, A.; et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 2014, 96, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Zaleski, A.L.; Taylor, B.A.; Thompson, P.D. Coenzyme Q10 as Treatment for Statin-Associated Muscle Symptoms—A Good Idea, but…. Adv. Nutr. 2018, 9, 519S–523S. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Wierzbicki, A.S. Statins, Muscle Disease and Mitochondria. J. Clin. Med. 2017, 6, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenson, R.S. Current overview of statin-induced myopathy. Am. J. Med. 2004, 116, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.M.; Thompson, P.D.; Catapano, A.L.; Stasiolek, M.; Fabis, J.; Muntner, P.; Serban, M.-C.; Banach, M. Statin-associated myopathy and the quest for biomarkers: Can we effectively predict statin-associated muscle symptoms? Drug Discov. Today 2017, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Scripko, P.D.; Amato, A.A.; Puig, A. Mystery case: A 63-year-old man with progressive proximal pain and weakness. Neurology 2014, 82, e26–e29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Timilsina, B.; Adhikari, J.; Parajuli, P.; Dhital, R.; Tachamo, N. Statin-induced necrotizing autoimmune myopathy: An extremely rare adverse effect from statin use. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Mohassel, P.; Mammen, A.L. Anti-HMGCR Myopathy. J. Neuromuscul. Dis. 2018, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Attardo, S.; Musumeci, O.; Velardo, D.; Toscano, A. Statins Neuromuscular Adverse Effects. Int. J. Mol. Sci. 2022, 23, 8364. [Google Scholar] [CrossRef] [PubMed]
- Forfar, J.C.; Brown, G.J.; Cull, R.E. Proximal myopathy during beta-blockade. Br. Med. J. 1979, 2, 1331–1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teicher, A.; Rosenthal, T.; Kissin, E.; Sarova, I. Labetalol-induced toxic myopathy. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 1824–1825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willis, J.K.; Tilton, A.H.; Harkin, J.C.; Boineau, F.G. Reversible myopathy due to labetalol. Pediatr. Neurol. 1990, 6, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Watanabe, N.; Hashimoto, J.; Nishiyama, A.; Sakuma, H.; Omata, K.; Abe, K.; Sekino, H. Muscle cramps and elevated serum creatine phosphokinase levels induced by beta-adrenoceptor blockers. Eur. J. Clin. Pharmacol. 1995, 48, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fernando Roth, R.; Itabashi, H.; Louie, J.; Anderson, T.; Narahara, K.A. Amiodarone toxicity: Myopathy and neuropathy. Am. Heart J. 1990, 119, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Pulipaka, U.; Lacomis, D.; Omalu, B. Amiodarone-induced neuromyopathy: Three cases and a review of the literature. J. Clin. Neuromuscul. Dis. 2002, 3, 97–105. [Google Scholar] [CrossRef]
- Meier, C.; Kauer, B.; Müller, U.; Ludin, H.P. Neuromyopathy during chronic amiodarone treatment. A case report. J. Neurol. 1979, 220, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Clouston, P.D.; Donnelly, P.E. Acute necrotising myopathy associated with amiodarone therapy. Aust. N. Z. J. Med. 1989, 19, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Harper, C.M.; St Louis, E.K.; Silber, M.H.; Josephs, K.A. Amiodarone-associated neuromyopathy: A report of four cases. Eur. J. Neurol. 2012, 19, e50–e51. [Google Scholar] [CrossRef] [PubMed]
- Kuncl, R.W.; Duncan, G.; Watson, D.; Alderson, K.; Rogawski, M.A.; Peper, M. Colchicine myopathy and neuropathy. N. Engl. J. Med. 1987, 316, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Figarella-Branger, D.; Alla, P.; Harlé, J.R.; Pellissier, J.F. Colchicine myopathy: A vacuolar myopathy with selective type I muscle fiber involvement. An immunohistochemical and electron microscopic study of two cases. Acta Neuropathol. 2002, 103, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Polsonetti, B.W.; Joy, S.D.; Laos, L.F. Steroid-induced myopathy in the ICU. Ann. Pharmacother. 2002, 36, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.; Dive, A.; Brucher, J.M.; Bisteau, M.; Dangoisse, M.; Deltombe, T. Acute corticosteroid myopathy in intensive care patients. Muscle Nerve 1997, 20, 1371–1380. [Google Scholar] [CrossRef]
- Askari, A.; Vignos, P.J., Jr.; Moskowitz, R.W. Steroid myopathy in connective tissue disease. Am. J. Med. 1976, 61, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W.; Ramsay, D.A.; Saly, V.; Shelley, S.; Moffatt, S. Acute necrotizing myopathy of intensive care: Electrophysiological studies. Muscle Nerve 1994, 17, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kanda, F.; Okuda, S.; Matsushita, T.; Takatani, K.; Kimura, K.I.; Chihara, K. Steroid myopathy: Pathogenesis and effects of growth hormone and insulin-like growth factor-I administration. Horm. Res. 2001, 56 (Suppl. S1), 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hollister, J.R. The untoward effects of steroid treatment on the musculoskeletal system and what to do about them. J. Asthma 1992, 29, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Faludi, G.; Gotlieb, J.; Meyers, J. Factors influencing the development of steroid-induced myopathies. Ann. N. Y. Acad. Sci. 1966, 138, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Horber, F.F.; Scheidegger, J.R.; Grünig, B.E.; Frey, F.J. Evidence that prednisone-induced myopathy is reversed by physical training. J. Clin. Endocrinol. Metab. 1985, 61, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Mammen, A.L. Toxic myopathies. Continuum 2013, 19, 1634–1649. [Google Scholar] [CrossRef]
- Naddaf, E.; Paul, P.; AbouEzzeddine, O.F. Chloroquine and Hydroxychloroquine Myopathy: Clinical Spectrum and Treatment Outcomes. Front. Neurol. 2021, 11, 616075. [Google Scholar] [CrossRef] [PubMed]
- Biguetti, C.C.; Junior, J.F.S.; Fiedler, M.W.; Marrelli, M.T.; Brotto, M. The toxic effects of chloroquine and hydroxychloroquine on skeletal muscle: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6589. [Google Scholar] [CrossRef] [PubMed]
- Casado, E.; Gratacós, J.; Tolosa, C.; Martínez, J.M.; Ojanguren, I.; Ariza, A.; Real, J.; Sanjuán, A.; Larrosa, M. Antimalarial myopathy: An underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann. Rheum. Dis. 2006, 65, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.L.; Ewing-Wilson, D.; Chou, S.M.; Mitsumoto, H.; Hanson, M.; Shirey, E.; Ratliff, N.B. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am. J. Med. 1987, 82, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Neville, H.E.; Maunder-Sewry, C.A.; McDougall, J.; Sewell, J.R.; Dubowitz, V. Chloroquine-induced cytosomes with curvilinear profiles in muscle. Muscle Nerve 1979, 2, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Solá, J.; Campistol, J.; Casademont, J.; Grau, J.M.; Urbano-Márquez, A. Reversible cyclosporin myopathy. Lancet 1990, 335, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Arellano, F.; Krupp, P. Muscular disorders associated with cyclosporin. Lancet 1991, 337, 915. [Google Scholar] [CrossRef] [PubMed]
- Breil, M.; Chariot, P. Muscle disorders associated with cyclosporine treatment. Muscle Nerve 1999, 22, 1631–1636. [Google Scholar] [CrossRef]
- Peters, B.S.; Winer, J.; Landon, D.N.; Stotter, A.; Pinching, A.J. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q. J. Med. 1993, 86, 5–15. [Google Scholar] [PubMed]
- Dalakas, M.C.; Illa, I.; Pezeshkpour, G.H.; Laukaitis, J.P.; Cohen, B.; Griffin, J.L. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 1990, 322, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Mhiri, C.; Baudrimont, M.; Bonne, G.; Degoul, F.; Marsac, C.; Roullet, E.; Gherardi, R. Zidovudine myopathy: A distinctive disorder associated with mitochondrial dysfunction. Ann. Neurol. 1991, 29, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Chariot, P.; Gherardi, R. Partial cytochrome c oxidase deficiency and cytoplasmic bodies in patients with zidovudine myopathy. Neuromuscul. Disord. 1991, 1, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Arnaudo, E.; Dalakas, M.; Shanske, S.; Moraes, C.T.; DiMauro, S.; Schon, E.A. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 1991, 337, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J., Jr.; McGuire, J.L.; Ochoa, J. Penicillamine-induced myositis in rheumatoid arthritis. Muscle Nerve 1981, 4, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Halla, J.T.; Fallahi, S.; Koopman, W.J. Penicillamine-induced myositis. Observations and unique features in two patients and review of the literature. Am. J. Med. 1984, 77, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, T.H.; Peck, K.R.; Song, Y.W. A case of polymyositis in a patient with primary biliary cirrhosis treated with D-penicillamine. Korean J. Intern. Med. 1993, 8, 46–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velcheti, V.; Schalper, K. Basic Overview of Current Immunotherapy Approaches in Cancer. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Liewluck, T.; Kao, J.C.; Mauermann, M.L. PD-1 Inhibitor-associated Myopathies: Emerging Immune-mediated Myopathies. J. Immunother. 2018, 41, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Maisonobe, T.; Knauss, S.; Salem, O.B.H.; Hervier, B.; Auré, K.; Szwebel, T.-A.; Kramkimel, N.; Lethrosne, C.; Bruch, J.-F.; et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018, 91, e985–e994, Erratum in Neurology 2019, 93, 280. [Google Scholar] [CrossRef] [PubMed]
- Anquetil, C.; Salem, J.E.; Lebrun-Vignes, B.; Johnson, D.B.; Mammen, A.L.; Stenzel, W.; Léonard-Louis, S.; Benveniste, O.; Moslehi, J.J.; Allenbach, Y. Immune Checkpoint Inhibitor-Associated Myositis: Expanding the Spectrum of Cardiac Complications of the Immunotherapy Revolution. Circulation 2018, 138, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728, Erratum in JAMA Oncol. 2018, 4, 1792. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Mochizuki, H.; Mochida, K.; Shiomi, K.; Amano, M.; Nakazato, M. A Case of Nivolumab-Induced Severe Mononeuropathy Multiplex and Rhabdomyolysis. Case Rep. Med. 2017, 2017, 1093858. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Otani, Y.; et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Finckh, A.; Bingham, C.O.; Visser, K.; Leipe, J.; Schulze-Koops, H.; Choy, E.H.; Benesova, K.; Radstake, T.; Cope, A.P.; et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann. Rheum. Dis. 2021, 80, 36–48. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur. J. Cancer 2019, 106, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, R.; Burel, S.L.; Dunogeant, L.; Marabelle, A.; Hollebecque, A.; Besse, B.; Leary, A.; Voisin, A.-L.; Pontoizeau, C.; Coutte, L.; et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann. Rheum. Dis. 2017, 76, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Tayar, J.H.; Abdel-Wahab, N.; Suarez-Almazor, M.E. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin. Arthritis Rheum. 2019, 48, 736–740. [Google Scholar] [CrossRef] [PubMed]



| DIAGNOSING | Anamnesis: age, gender, onset, location, extent, triggering and relieving factors of pain, associated symptoms, past medical history, medications ↓ Physical examination: fever, body mass loss, decreased muscle strength, muscle atrophy or hypertrophy, fasciculations, myotonia, asymmetrical sensory loss, “tender points”, signs of arthritis, skin lesions ↓ Laboratory tests: ESR, CRP, blood count, ionogram, CK, AST, ALT, creatinine, LDH, TSH, cortisol, ANA with specification, RF, ACPA ↓ Additional examinations: X-ray of joints, MRI of muscles, electromyography, muscle biopsy, genetic tests↓ Multi-specialist cooperation is often necessary | |
| IDENTIFICATION OF ETIOLOGY (see Figure 1) | INFECTIONS DRUGS METABOLIC ENDOCRINOLOGICAL | INFLAMMATORY DISEASES NEUROPATHIES HEREDITARY MYOPATHIES PSYCHOSOMATIC |
| MANAGEMENT | Depends on etiology and cause:
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miernik, S.; Matusiewicz, A.; Olesińska, M. Drug-Induced Myopathies: A Comprehensive Review and Update. Biomedicines 2024, 12, 987. https://doi.org/10.3390/biomedicines12050987
Miernik S, Matusiewicz A, Olesińska M. Drug-Induced Myopathies: A Comprehensive Review and Update. Biomedicines. 2024; 12(5):987. https://doi.org/10.3390/biomedicines12050987
Chicago/Turabian StyleMiernik, Sebastian, Agata Matusiewicz, and Marzena Olesińska. 2024. "Drug-Induced Myopathies: A Comprehensive Review and Update" Biomedicines 12, no. 5: 987. https://doi.org/10.3390/biomedicines12050987
APA StyleMiernik, S., Matusiewicz, A., & Olesińska, M. (2024). Drug-Induced Myopathies: A Comprehensive Review and Update. Biomedicines, 12(5), 987. https://doi.org/10.3390/biomedicines12050987






