Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration
Abstract
:1. Introduction: Setting the Scene
2. Separation Performance of HF OSN Membranes
2.1. Separation Mechanism: The Swelling Challenge
2.2. Performance Characterization

3. Fabrication of Interfacially Polymerized HF OSN Membranes
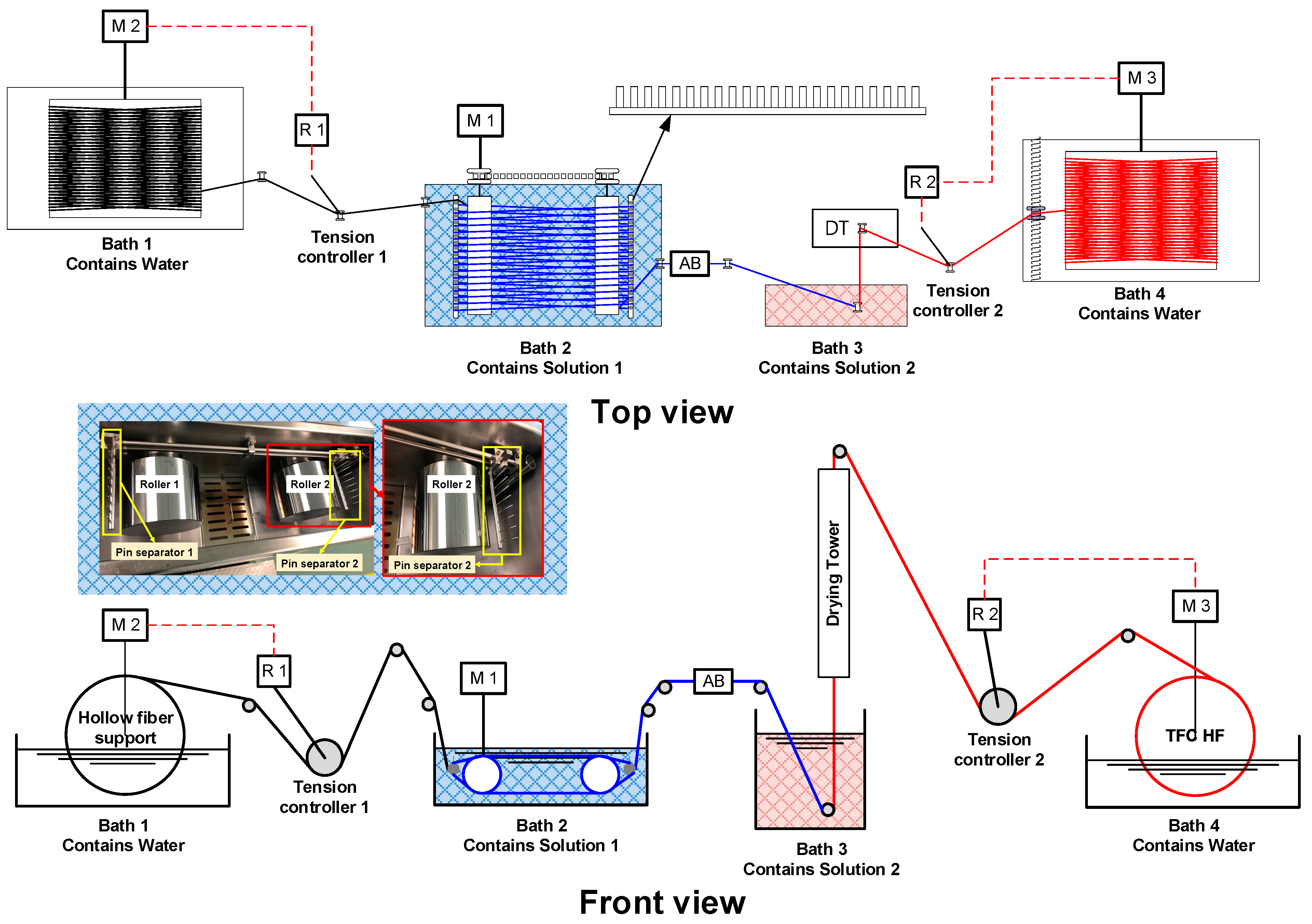
3.1. Interfacial Polymerization Fundamentals
3.2. Porous Support
3.3. Selective Layer
3.4. Post-Treatment Methods
3.4.1. Crosslinking
3.4.2. Solvent Activation
3.4.3. Thermal Annealing
3.5. Module Configuration
4. Greener Interfacially Polymerized HF OSN Membranes
4.1. Greener Solvents
4.2. Biopolymers
4.3. Recyclable Polymers
4.4. Bio-Based Monomers
4.5. Alternative Fabrication Methods
5. Challenges and Commercial Viability
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cseri, L.; Razali, M.; Pogany, P.; Szekely, G. Organic Solvents in Sustainable Synthesis and Engineering. In Green Chemistry: An Inclusive Approach; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Bae, J. Organic solvent nanofiltration in pharmaceutical industry. Sep. Purif. Rev. 2015, 44, 157–182. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Buonomenna, M.G.; Golemme, G.; Perrotta, E. Membrane operations for industrial applications. In Advances in Chemical Engineering; Nawaz, Z., Naveed, S., Eds.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Liu, C.; Dong, G.Y.; Tsuru, T.; Matsuyama, H. Organic solvent reverse osmosis membranesfor organic liquid mixture separation: A review. J. Membr. Sci. 2021, 620, 118882. [Google Scholar] [CrossRef]
- Chau, J.; Sirkar, K.K. Organic solvent mixture separation during reverse osmosis and nanofiltration by a perfluorodioxole copolymer membrane. J. Membr. Sci. 2021, 618, 118663. [Google Scholar] [CrossRef]
- Cui, Y.; Chung, T.S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Vandezande, P.; Gevers, L.E.M.; Vankelecom, I.F.J. Solvent Resistant Nanofiltration: Separating on a Molecular Level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef]
- Asadi Tashvigh, A.; Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.S. 110th Anniversary: Selection of Cross-Linkers and Cross-Linking Procedures for the Fabrication of Solvent-Resistant Nanofiltration Membranes: A Review. Ind. Eng. Chem. Res. 2019, 58, 10678–10691. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Wang, E.; Su, B. Hollow Fiber Membrane for Organic Solvent Nanofiltration: A Mini Review. Membranes 2022, 12, 995. [Google Scholar] [CrossRef]
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.Y.; Chung, T.S. Recent Progress of Organic Solvent Nanofiltration Membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Lau, H.S.; Lau, S.K.; Soh, L.S.; Hong, S.U.; Gok, X.Y.; Yi, S.; Yong, W.F. State-of-the-Art Organic-and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes 2022, 12, 539. [Google Scholar] [CrossRef]
- Scholz, M.; Wessling, M.; Balster, J. Chapter 5. Design of Membrane Modules for Gas Separations. In Membrane Engineering for the Treatment of Gases; Drioli, E., Barbieri, G., Eds.; RSC: London, UK, 2011. [Google Scholar]
- Liu, T.; Qin, Z.; Liu, Q.; Li, X.; Liu, Y.; An, Q.F.; Guo, H. In Situ Growth of a Tubular MoS2membrane on a Ceramic Tube with Improved Organic Solvent Nanofiltration Performance. Mater. Chem. Front. 2021, 5, 3184–3191. [Google Scholar] [CrossRef]
- Merlet, R.; Winnubst, L.; Nijmeijer, A.; Amirilargani, M.; Sudhölter, E.J.R.; de Smet, L.C.P.M.; Salvador Cob, S.; Vandezande, P.; Dorbec, M.; Sluijter, S.; et al. Comparing the Performance of Organic Solvent Nanofiltration Membranes in Non-Polar Solvents. Chem. Ing. Tech. 2021, 93, 1389–1395. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Wang, K.Y.; Weber, M.; Chung, T.S. Polybenzimidazoles (PBIs) and State-of-the-Art PBI Hollow Fiber Membranes for Water, Organic Solvent and Gas Separations: A Review. J. Mater. Chem. A Mater. 2022, 10, 8687–8718. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Tarabara, V.V. Encyclopedia of Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tul Muntha, S.; Kausar, A.; Siddiq, M. Advances in Polymeric Nanofiltration Membrane: A Review. Polym. Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Lim, S.K.; Goh, K.; Bae, T.H.; Wang, R. Polymer-Based Membranes for Solvent-Resistant Nanofiltration: A Review. Chin. J. Chem. Eng. 2017, 25, 1653–1675. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Li, S.; Van der Bruggen, B. Interfacially Polymerized Thin-Film Composite Membranes for Organic Solvent Nanofiltration. Adv. Mater. Interfaces 2021, 8, 2001671. [Google Scholar] [CrossRef]
- Szekely, G.; Jimenez-Solomon, M.F.; Marchetti, P.; Kim, J.F.; Livingston, A.G. Sustainability Assessment of Organic Solvent Nanofiltration: From Fabrication to Application. Green Chem. 2014, 16, 4440–4473. [Google Scholar] [CrossRef]
- Peeva, L.G.; Marchetti, P.; Livingston, A.G. Nanofiltration Operations in Nonaqueous Systems. In Comprehensive Membrane Science and Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2. [Google Scholar]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and Applications of Hollow Fiber Nanofiltration Membranes: A Review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, W.A.; Cornelissen, E.R.; de Vos, W.M. Hollow Fiber Nanofiltration: From Lab-Scale Research to Full-Scale Applications. J. Membr. Sci. 2023, 669, 121234. [Google Scholar] [CrossRef]
- Marchetti, P.; Peeva, L.; Livingston, A. The Selectivity Challenge in Organic Solvent Nanofiltration: Membrane and Process Solutions. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M. Relation of Structure to Chemical Properties. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Buonomenna, M.G.; Golemme, G.; Jansen, J.C.; Choi, S.-H. Asymmetric PEEKWC membranes for treatment of organic solvent solutions. J. Membr. Sci. 2011, 368, 144–149. [Google Scholar] [CrossRef]
- Xu, Y.; You, F.; Sun, H.; Shao, L. Realizing mussel-inspired polydopamine selective layer with strong solvent resistance in nanofiltration toward sustainable reclamation. ACS Sustain. Chem. Eng. 2017, 5, 5520–5528. [Google Scholar] [CrossRef]
- Kosaraju, P.B.; Sirkar, K.K. Interfacially polymerized thin film composite membranes on microporous polypropylene supports for solvent-resistant nanofiltration. J. Membr. Sci. 2008, 321, 155–161. [Google Scholar] [CrossRef]
- Sun, S.P.; Chung, T.S.; Lu, K.J.; Chan, S.Y. Enhancement of flux and solvent stability of Matrimid® thin-film composite membranes for organic solvent nanofiltration. AIChE J. 2014, 60, 3623–3633. [Google Scholar] [CrossRef]
- Ebert, K.; Cuperus, F.P. Solvent resistant nanofiltration in edible oil processing. Membr. Technol. 1999, 107, 5–8. [Google Scholar]
- Tarleton, E.S.; Robinson, J.P.; Salman, M. Solvent-induced swelling of membranes—Measurements and influence in nanofiltration. J. Membr. Sci. 2006, 280, 442–451. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.S.; Rajagopalan, R. Dehydration of tetrafluoropropanol (TFP) by pervaporation via novel PBI/BTDA-TDI/MDI co-polyimide (P84) dual-layer hollow fiber membranes. J. Membr. Sci. 2007, 287, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.; Gruender, M.; Chung, T.S. Pervaporation dehydration of ethylene glycol through polybenzimidazole (PBI)-based membranes. 1. Membrane fabrication. J. Membr. Sci. 2010, 363, 149–159. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Chung, T.S. Robust polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration. J. Membr. Sci. 2019, 572, 580–587. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Chung, T.S. A novel crosslinking technique towards the fabrication of high-flux polybenzimidazole (PBI) membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2019, 209, 182–192. [Google Scholar] [CrossRef]
- Xing, D.Y.; Chan, S.Y.; Chung, T.S. The ionic liquid [EMIM] OAc as a solvent to fabricate stable polybenzimidazole membranes for organic solvent nanofiltration. Green Chem. 2014, 16, 1383–1392. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Kumbharkar, S.C.; Kim, J.F.; Bhole, Y.; Livingston, A.G. Beyond polyimide: Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN) in harsh environments. J. Membr. Sci. 2014, 457, 62–72. [Google Scholar] [CrossRef]
- Chen, D.; Yu, S.; Yang, M.; Li, D.; Li, X. Solvent resistant nanofiltration membranes based on crosslinked polybenzimidazole. RSC Adv. 2016, 6, 16925–16932. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Chung, T.S. Facile fabrication of solvent resistant thin film composite membranes by interfacial crosslinking reaction between polyethylenimine and dibromo-p-xylene on polybenzimidazole substrates. J. Membr. Sci. 2018, 560, 115–124. [Google Scholar] [CrossRef]
- Chen, D.; Yan, C.; Li, X.; Liu, L.; Wu, D.; Li, X. A highly stable PBI solvent resistant nanofiltration membrane prepared via versatile and simple crosslinking process. Sep. Purif. Technol. 2019, 224, 15–22. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, G.M.; Wang, K.Y.; Lai, J.Y.; Chung, T.S. Employing a green cross-linking method to fabricate polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2021, 255, 117702. [Google Scholar] [CrossRef]
- Quan, Q.; Xiao, C.; Liu, H.; Huang, Q.; Zhao, W.; Hu, X.; Huan, G. Preparation and characterization of braided tube reinforced polyacrylonitrile hollow fiber membranes. J. Appl. Polym. Sci. 2015, 132, 41795. [Google Scholar] [CrossRef]
- Fan, Z.; Xiao, C.; Liu, H.; Huang, Q.; Zhao, J. Structure design and performance study on braid-reinforced cellulose acetate hollow fiber membranes. J. Membr. Sci. 2015, 486, 248–256. [Google Scholar] [CrossRef]
- Zhao, B.; Min Shi, G.; Lai, J.H.; Chung, T.S. Braid-reinforced polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2022, 290, 120811. [Google Scholar] [CrossRef]
- Böcking, A.; Koleva, V.; Wind, J.; Thiermeyer, Y.; Blumenschein, S.; Goebel, R.; Skiborowski, M.; Wessling, M. Can the Variance in Membrane Performance Influence the Design of Organic Solvent Nanofiltration Processes? J. Membr. Sci. 2019, 575, 217–228. [Google Scholar] [CrossRef]
- White, L.S. Transport Properties of a Polyimide Solvent Resistant Nanofiltration Membrane. J. Membr. Sci. 2002, 205, 191–202. [Google Scholar] [CrossRef]
- Low, Z.X.; Patterson, D.A.; Patterson, D.A. Molecular Weight Cut-off Determination of Organic Solvent Nanofiltration Membranes Using Poly(Propylene Glycol). J. Membr. Sci. 2017, 526, 221–228. [Google Scholar]
- See Toh, Y.H.; Loh, X.X.; Li, K.; Bismarck, A.; Livingston, A.G. In Search of a Standard Method for the Characterisation of Organic Solvent Nanofiltration Membranes. J. Membr. Sci. 2007, 291, 120–125. [Google Scholar] [CrossRef]
- Silva, P.; Han, S.; Livingston, A.G. Solvent Transport in Organic Solvent Nanofiltration Membranes. J. Membr. Sci. 2005, 262, 49–59. [Google Scholar] [CrossRef]
- Ohkame, T.; Minegishi, K.; Sugihara, H.; Nakagawa, K.; Shintani, T.; Matsuyama, H.; Yoshioka, T. Hollow-Fiber Ro Membranes Fabricated via Adsorption of Low-Charge Poly(Vinyl Alcohol) Copolymers. Membranes 2021, 11, 981. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.B.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 21840–21856. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. Hollow Fiber Membrane-Based Separation Technology: Performance and Design Perspectives. In Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Sun, S.P.; Chan, S.Y.; Chung, T.S. A Slow-Fast Phase Separation (SFPS) Process to Fabricate Dual-Layer Hollow Fiber Substrates for Thin-Film Composite (TFC) Organic Solvent Nanofiltration (OSN) Membranes. Chem. Eng. Sci. 2015, 129, 232–242. [Google Scholar] [CrossRef]
- Goh, K.S.; Chong, J.Y.; Chen, Y.; Fang, W.; Bae, T.H.; Wang, R. Thin-Film Composite Hollow Fibre Membrane for Low Pressure Organic Solvent Nanofiltration. J. Membr. Sci. 2020, 597, 117760. [Google Scholar] [CrossRef]
- Goh, K.S.; Chen, Y.; Chong, J.Y.; Bae, T.H.; Wang, R. Thin Film Composite Hollow Fibre Membrane for Pharmaceutical Concentration and Solvent Recovery. J. Membr. Sci. 2021, 621, 119008. [Google Scholar] [CrossRef]
- Su, J.; Lv, X.; Li, S.; Jiang, Y.; Liu, S.; Zhang, X.; Li, H.; Su, B. High Separation Performance Thin Film Composite and Thin Film Nanocomposite Hollow Fiber Membranes via Interfacial Polymerization for Organic Solvent Nanofiltration. Sep. Purif. Technol. 2022, 278, 119567. [Google Scholar] [CrossRef]
- Gao, Z.F.; Liu, J.; Chung, T.S. Rapid In-Situ Growth of Covalent Organic Frameworks on Hollow Fiber Substrates with Janus-like Characteristics for Efficient Organic Solvent Nanofiltration. Sep. Purif. Technol. 2022, 294, 121166. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High Flux Thin Film Nanocomposite Membrane Incorporated with Functionalized TiO2@reduced Graphene Oxide Nanohybrids for Organic Solvent Nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Song, C.; Tang, S.; Yue, S.; Cui, Z.; Du, X.; Jiang, T.; He, B.; Li, J. Design of Microstructure for Hollow Fiber Loose Nanofiltration Separation Layer and Its Compactness-Tailoring Mechanism. J. Hazard. Mater. 2022, 421, 126800. [Google Scholar] [CrossRef] [PubMed]
- Francis, V.N.; Chong, J.Y.; Yang, G.; Che, L.; Wang, R. Robust Polyamide-PTFE Hollow Fibre Membranes for Harsh Organic Solvent Nanofiltration. Chem. Eng. J. 2023, 452, 139333. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Fang, C.; Zhang, L.; Zhu, L. Controllable Thermal Annealing of Polyimide Membranes for Highly-Precise Organic Solvent Nanofiltration. J. Membr. Sci. 2022, 643, 120013. [Google Scholar] [CrossRef]
- Baker, R.W. Membranes and Modules. In Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Chung, T.S.; Hu, X. Effect of Air-Gap Distance on the Morphology and Thermal Properties of Polyethersulfone Hollow Fibers. J. Appl. Polym. Sci. 1997, 66, 1067–1077. [Google Scholar] [CrossRef]
- Chung, T.S.; Teoh, S.K.; Lau, W.W.Y.; Srinivasan, M.P. Effect of Shear Stress within the Spinneret on Hollow Fiber Membrane Morphology and Separation Performance. Ind. Eng. Chem. Res. 1998, 37, 3930–3938. [Google Scholar] [CrossRef]
- Qin, J.J.; Wang, R.; Chung, T.S. Investigation of Shear Stress Effect within a Spinneret on Flux, Separation and Thermomechanical Properties of Hollow Fiber Ultrafiltration Membranes. J. Membr. Sci. 2000, 175, 197–213. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, S.; Xu, S.; Tian, L.; Su, B.; Han, L.; Mandal, B. Fundamental Understanding on the Preparation Conditions of High-Performance Polyimide-Based Hollow Fiber Membranes for Organic Solvent Nanofiltration (OSN). Sep. Purif. Technol. 2021, 254, 117600. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Feng, R.; Wang, W.J.; Sun, Y.X.; Tao, S.N.; Wang, Y.M.; Chen, Y.F.; Fu, Z.J.; Cao, X.L.; Sun, S.P.; et al. Robust Braid Reinforced Hollow Fiber Membranes for Organic Solvent Nanofiltration (OSN). Adv. Membr. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; McCutcheon, J.R. Braid-Reinforced Thin Film Composite Hollow Fiber Nanofiltration Membranes. J. Membr. Sci. 2019, 585, 109–114. [Google Scholar] [CrossRef]
- He, T.; Mulder, M.H.V.; Strathmann, H.; Wessling, M. Preparation of Composite Hollow Fiber Membranes: Co-Extrusion of Hydrophilic Coatings onto Porous Hydrophobic Support Structures. J. Membr. Sci. 2002, 207, 143–156. [Google Scholar] [CrossRef]
- Li, X.M.; Ji, Y.; Yin, Y.; Zhang, Y.Y.; Wang, Y.; He, T. Origin of Delamination/Adhesion in Polyetherimide/Polysulfone Co-Cast Membranes. J. Membr. Sci. 2010, 352, 173–179. [Google Scholar] [CrossRef]
- Kopeć, K.K.; Dutczak, S.M.; Wessling, M.; Stamatialis, D.F. Chemistry in a Spinneret-On the Interplay of Crosslinking and Phase Inversion during Spinning of Novel Hollow Fiber Membranes. J. Membr. Sci. 2011, 369, 308–318. [Google Scholar] [CrossRef]
- Dutczak, S.M.; Tanardi, C.R.; Kopeć, K.K.; Wessling, M.; Stamatialis, D. “Chemistry in a Spinneret” to Fabricate Hollow Fibers for Organic Solvent Filtration. Sep. Purif. Technol. 2012, 86, 183–189. [Google Scholar] [CrossRef]
- Emonds, S.; Roth, H.; Wessling, M. Chemistry in a Spinneret—Formation of Hollow Fiber Membranes with a Cross-Linked Polyelectrolyte Separation Layer. J. Membr. Sci. 2020, 612, 118325. [Google Scholar] [CrossRef]
- Sun, S.P.; Chan, S.Y.; Xing, W.; Wang, Y.; Chung, T.S. Facile Synthesis of Dual-Layer Organic Solvent Nanofiltration (OSN) Hollow Fiber Membranes. ACS Sustain. Chem. Eng. 2015, 3, 3019–3023. [Google Scholar] [CrossRef]
- Soroko, I.; Livingston, A. Impact of TiO2 Nanoparticles on Morphology and Performance of Crosslinked Polyimide Organic Solvent Nanofiltration (OSN) Membranes. J. Membr. Sci. 2009, 343, 189–198. [Google Scholar] [CrossRef]
- Davood Abadi Farahani, M.H.; Chung, T.S. Solvent Resistant Hollow Fiber Membranes Comprising P84 Polyimide and Amine-Functionalized Carbon Nanotubes with Potential Applications in Pharmaceutical, Food, and Petrochemical Industries. Chem. Eng. J. 2018, 345, 174–185. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; Weyd, M.; McCutcheon, J.R. Ceramic-Supported Thin Film Composite Membrane for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 563, 857–863. [Google Scholar] [CrossRef]
- Huang, W.Z.; Lin, F.; Lee, S.L.; Tao, F.T.; Tung, K.L. Fabrication of Microporous Polyamide Selective Layer on Macroporous Ceramic Hollow Fibers via Direct Interfacial Polymerization for Nanofiltration Applications. J. Membr. Sci. 2022, 658, 120710. [Google Scholar] [CrossRef]
- Wang, X.L.; Wei, J.F.; Dai, Z.; Zhao, K.Y.; Zhang, H. Preparation and Characterization of Negatively Charged Hollow Fiber Nanofiltration Membrane by Plasma-Induced Graft Polymerization. Desalination 2012, 286, 138–144. [Google Scholar] [CrossRef]
- Xu, H.M.; Wei, J.F.; Wang, X.L. Nanofiltration Hollow Fiber Membranes with High Charge Density Prepared by Simultaneous Electron Beam Radiation-Induced Graft Polymerization for Removal of Cr(VI). Desalination 2014, 346, 122–130. [Google Scholar] [CrossRef]
- Aerts, S.; Vanhulsel, A.; Buekenhoudt, A.; Weyten, H.; Kuypers, S.; Chen, H.; Bryjak, M.; Gevers, L.E.M.; Vankelecom, I.F.J.; Jacobs, P.A. Plasma-Treated PDMS-Membranes in Solvent Resistant Nanofiltration: Characterization and Study of Transport Mechanism. J. Membr. Sci. 2006, 275, 212–219. [Google Scholar] [CrossRef]
- Korikov, A.P.; Kosaraju, P.B.; Sirkar, K.K. Interfacially Polymerized Hydrophilic Microporous Thin Film Composite Membranes on Porous Polypropylene Hollow Fibers and Flat Films. J. Membr. Sci. 2006, 279, 588–600. [Google Scholar] [CrossRef]
- Vanhook, S.J.; Schatz, M.F.; Swift, J.B.; McCormick, W.D.; Swinney, H.L. Long-Wavelength Surface-Tension-Driven Bénard Convection: Experiment and Theory. J. Fluid Mech. 1997, 345, 45–78. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 Nm Polyamide Nanofilms with Ultrafast Solvent Transport for Molecular Separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Feng, W.; Zhu, L.; Zhang, L. PIM-1 Pore-Filled Thin Film Composite Membranes for Tunable Organic Solvent Nanofiltration. J. Membr. Sci. 2020, 601, 117951. [Google Scholar] [CrossRef]
- Fritsch, D.; Merten, P.; Heinrich, K.; Lazar, M.; Priske, M. High Performance Organic Solvent Nanofiltration Membranes: Development and Thorough Testing of Thin Film Composite Membranes Made of Polymers of Intrinsic Microporosity (PIMs). J. Membr. Sci. 2012, 401–402, 222–231. [Google Scholar] [CrossRef]
- Yang, E.; Liang, Y.; Yanar, N.; Kim, M.; Park, H.; Choi, H. Intermolecular Cross-Linked Polymer of Intrinsic Microporosity-1 (PIM-1)-Based Thin-Film Composite Hollow Fiber Membrane for Organic Solvent Nanofiltration. J. Membr. Sci. 2023, 671, 121370. [Google Scholar] [CrossRef]
- Gorgojo, P.; Karan, S.; Wong, H.C.; Jimenez-Solomon, M.F.; Cabral, J.T.; Livingston, A.G. Ultrathin Polymer Films with Intrinsic Microporosity: Anomalous Solvent Permeation and High Flux Membranes. Adv. Funct. Mater. 2014, 24, 4729–4737. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High Flux Thin Film Nanocomposite Membranes Based on Metal-Organic Frameworks for Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.K.; Falca, G.; Upadhya, L.; Abou-Hamad, E.; Batra, N.; Wang, S.; Musteata, V.; da Costa, P.M.; Nunes, S.P. Spray-Coated Graphene Oxide Hollow Fibers for Nanofiltration. J. Membr. Sci. 2020, 606, 118006. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Chen, J.; Ge, R.; Leng, W.; Dong, B.; Huang, J.; Gao, Y. A Novel 3D Covalent Organic Framework Membrane Grown on a Porous α-Al2O3 Substrate under Solvothermal Conditions. Chem. Commun. 2015, 51, 15562–15565. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, A.; Zhang, C.; Wang, Y. Growing Covalent Organic Frameworks on Porous Substrates for Molecule-Sieving Membranes with Pores Tunable from Ultra- to Nanofiltration. J. Membr. Sci. 2019, 576, 116–122. [Google Scholar] [CrossRef]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.S. Emerging Thin-Film Nanocomposite (TFN) Membranes for Reverse Osmosis: A Review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking Polyimides for Membrane Applications: A Review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Vanherck, K.; Cano-Odena, A.; Koeckelberghs, G.; Dedroog, T.; Vankelecom, I. A Simplified Diamine Crosslinking Method for PI Nanofiltration Membranes. J. Membr. Sci. 2010, 353, 135–143. [Google Scholar] [CrossRef]
- Guo, X.; Liu, D.; Han, T.; Huang, H.; Yang, Q.; Zhong, C. Preparation of Thin Film Nanocomposite Membranes with Surface Modified MOF for High Flux Organic Solvent Nanofiltration. AIChE J. 2017, 63, 1303–1312. [Google Scholar] [CrossRef]
- Lim, S.K.; Setiawan, L.; Bae, T.H.; Wang, R. Polyamide-Imide Hollow Fiber Membranes Crosslinked with Amine-Appended Inorganic Networks for Application in Solvent-Resistant Nanofiltration under Low Operating Pressure. J. Membr. Sci. 2016, 501, 152–160. [Google Scholar] [CrossRef]
- Tham, H.M.M.; Wang, K.Y.; Hua, D.; Japip, S.; Chung, T.S. From Ultrafiltration to Nanofiltration: Hydrazine Cross-Linked Polyacrylonitrile Hollow Fiber Membranes for Organic Solvent Nanofiltration. J. Membr. Sci. 2017, 542, 289–299. [Google Scholar] [CrossRef]
- Dutczak, S.M.; Cuperus, F.P.; Wessling, M.; Stamatialis, D.F. New Crosslinking Method of Polyamide-Imide Membranes for Potential Application in Harsh Polar Aprotic Solvents. Sep. Purif. Technol. 2013, 102, 142–146. [Google Scholar] [CrossRef]
- Wang, K.Y.; Li, B.; Chung, T.S. 3D-Macrocycles Impregnated Polybenzimidazole Hollow Fiber Membranes with Excellent Organic Solvent Resistance for Industrial Solvent Recovery. J. Membr. Sci. 2021, 638, 119678. [Google Scholar] [CrossRef]
- Loh, X.X.; Sairam, M.; Steinke, J.H.G.; Livingston, A.G.; Bismarck, A.; Li, K. Polyaniline Hollow Fibres for Organic Solvent Nanofiltration. Chem. Commun. 2008, 47, 6324–6326. [Google Scholar] [CrossRef]
- See Toh, Y.H.; Lim, F.W.; Livingston, A.G. Polymeric Membranes for Nanofiltration in Polar Aprotic Solvents. J. Membr. Sci. 2007, 301, 3–10. [Google Scholar] [CrossRef]
- Jimenez Solomon, M.F.; Bhole, Y.; Livingston, A.G. High Flux Membranes for Organic Solvent Nanofiltration (OSN)-Interfacial Polymerization with Solvent Activation. J. Membr. Sci. 2012, 423–424, 371–382. [Google Scholar] [CrossRef]
- Shin, M.G.; Park, S.H.; Kwon, S.J.; Kwon, H.E.; Park, J.B.; Lee, J.H. Facile Performance Enhancement of Reverse Osmosis Membranes via Solvent Activation with Benzyl Alcohol. J. Membr. Sci. 2019, 578, 220–229. [Google Scholar] [CrossRef]
- Mariën, H.; Vankelecom, I.F.J. Transformation of Cross-Linked Polyimide UF Membranes into Highly Permeable SRNF Membranes via Solvent Annealing. J. Membr. Sci. 2017, 541, 205–213. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Li, S.; Guo, Z.; Van Der Bruggen, B. Flexible Aliphatic-Aromatic Polyamide Thin Film Composite Membrane for Highly Efficient Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2020, 12, 31962–31974. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and Guiding the Phase Inversion Process for Synthesis of Solvent Resistant Nanofiltration Membranes. J. Appl. Polym. Sci. 2015, 132, 42130. [Google Scholar] [CrossRef]
- Lai, X.; Wang, C.; Wang, L.; Xiao, C. A Novel PPTA/PPy Composite Organic Solvent Nanofiltration (OSN) Membrane Prepared by Chemical Vapor Deposition for Organic Dye Wastewater Treatment. J. Water Process Eng. 2022, 45, 102533. [Google Scholar] [CrossRef]
- Tham, H.M.; Chung, T.S. One-Step Cross-Linking and Tannic Acid Modification of Polyacrylonitrile Hollow Fibers for Organic Solvent Nanofiltration. J. Membr. Sci. 2020, 610, 118294. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic Solvents in the Pharmaceutical Industry. Acta Pol. Pharm. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Li, Y.; Cao, B.; Li, P. Effects of Dope Compositions on Morphologies and Separation Performances of PMDA-ODA Polyimide Hollow Fiber Membranes in Aqueous and Organic Solvent Systems. Appl. Surf. Sci. 2019, 473, 1038–1048. [Google Scholar] [CrossRef]
- Jeon, S.; Nishitani, A.; Cheng, L.; Fang, L.F.; Kato, N.; Shintani, T.; Matsuyama, H. One-Step Fabrication of Polyamide 6 Hollow Fibre Membrane Using Non-Toxic Diluents for Organic Solvent Nanofiltration. RSC Adv. 2018, 8, 19879–19882. [Google Scholar] [CrossRef]
- Falca, G.; Musteata, V.E.; Behzad, A.R.; Chisca, S.; Nunes, S.P. Cellulose Hollow Fibers for Organic Resistant Nanofiltration. J. Membr. Sci. 2019, 586, 151–161. [Google Scholar] [CrossRef]
- McGuinness, E.K.; Zhang, F.; Ma, Y.; Lively, R.P.; Losego, M.D. Vapor Phase Infiltration of Metal Oxides into Nanoporous Polymers for Organic Solvent Separation Membranes. Chem. Mater. 2019, 31, 5509–5518. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Fu, Z.J.; Shao, D.D.; Lu, M.J.; Xia, Q.C.; Xiao, H.F.; Su, B.W.; Sun, S.P. Bridging the Miscibility Gap to Fabricate Delamination-Free Dual-Layer Nanofiltration Membranes via Incorporating Fluoro Substituted Aromatic Amine. J. Membr. Sci. 2020, 610, 118270. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.Y.; Li, S.; Tian, L.; Su, B. Fabrication of Polyimide-Based Hollow Fiber Membrane by Synergetic Covalent-Crosslinking Strategy for Organic Solvent Nanofiltration (OSN) Application. Sep. Purif. Technol. 2020, 241, 116751. [Google Scholar] [CrossRef]
- Kato, N.; Gonzales, R.R.; Nishitani, A.; Negi, Y.; Ono, T.; Matsuyama, H. Single-Step Preparation of Nanocomposite Polyamide 6 Hollow Fiber Membrane with Integrally Skinned Asymmetric Structure for Organic Solvent Nanofiltration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126538. [Google Scholar] [CrossRef]
- Junker, M.A.; de Vos, W.M.; Lammertink, R.G.H.; de Grooth, J. Bridging the gap between lab-scale and commercial dimensions of hollow fiber nanofiltration membranes. J. Membr. Sci. 2021, 624, 119100. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Nano-Enhanced and Nanostructured Polymer-Based Membranes for Energy Applications; Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Labban, O.; Chong, T.H.; Lienhard, J.H. Design and modeling of novel low-pressure nanofiltration hollow fiber modules for water softening and desalination pretreatment. Desalination 2018, 439, 58–72. [Google Scholar] [CrossRef]
- Jährig, J.; Vredenbregt, L.; Wicke, D.; Miehe, U.; Sperlich, A. Capillary nanofiltration under anoxic conditions as post-treatment after bank filtration. Water 2018, 10, 1599. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Nguyen Thi, H.Y.; Kim, S.; Duy Nguyen, B.T.; Lim, D.; Kumar, S.; Lee, H.; Szekely, G.; Kim, J.F. Closing the Sustainable Life Cycle Loop of Membrane Technology via a Cellulose Biomass Platform. ACS Sustain. Chem. Eng. 2022, 10, 2532–2544. [Google Scholar] [CrossRef]
- Lin, S.; Semiao, A.C.; Zhang, Y.; Lu, S.; Hon Lau, C. Interfacial polymerization using biobased solvents and their application as desalination and organic solvent nanofiltration membranes. J. Membr. Sci. 2024, 692, 122281. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Alcantara, A.R.; Dominguez de Maria, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.H.; Wong, L.L.; Tan, J.; Isoni, V.; Sharratt, P. Synthesis of 2-methyl tetrahydrofuran from various lignocellulosic feedstocks: Sustainability assessment via Lca. Resour. Conserv. Recycl. 2015, 95, 174–182. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards Cleaner PolarClean: Efficient Synthesis and Extended Applications of the Polar Aprotic Solvent Methyl 5-(Dimethylamino)-2-Methyl-5-Oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Hardian, R.; Cywar, R.M.; Chen, E.Y.X.; Szekely, G. Sustainable Nanofiltration Membranes Based on Biosourced Fully Recyclable Polyesters and Green Solvents. J. Membr. Sci. Lett. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Ong, C.; Falca, G.; Huang, T.; Liu, J.; Manchanda, P.; Chisca, S.; Nunes, S.P. Green Synthesis of Thin-Film Composite Membranes for Organic Solvent Nanofiltration. ACS Sustain. Chem. Eng. 2020, 8, 11541–11548. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. Use of γ-Valerolactone and Glycerol Derivatives as Bio-Based Renewable Solvents for Membrane Preparation. Green Chem. 2019, 21, 1054–1064. [Google Scholar] [CrossRef]
- Dargo, G.; Kis, D.; Gede, M.; Kumar, S.; Kupai, J.; Szekely, G. MeSesamol, a Bio-Based and Versatile Polar Aprotic Solvent for Organic Synthesis and Depolymerization. Chem. Eng. J. 2023, 471, 144365. [Google Scholar] [CrossRef]
- Park, S.H.; Yang, C.; Ayaril, N.; Szekely, G. Solvent-Resistant Thin-Film Composite Membranes from Biomass-Derived Building Blocks: Chitosan and 2,5-Furandicarboxaldehyde. ACS Sustain. Chem. Eng. 2022, 10, 998–1007. [Google Scholar] [CrossRef]
- Alqadhi, N.; Abdellah, M.H.; Nematulloev, S.; Mohammed, O.F.; Abdulhamid, M.A.; Szekely, G. Solution-Processable Poly(Ether-Ether-Ketone) Membranes for Organic Solvent Nanofiltration: From Dye Separation to Pharmaceutical Purification. Sep. Purif. Technol. 2024, 328, 125072. [Google Scholar] [CrossRef]
- Yang, C.; Topuz, F.; Park, S.H.; Szekely, G. Biobased Thin-Film Composite Membranes Comprising Priamine-Genipin Selective Layer on Nanofibrous Biodegradable Polylactic Acid Support for Oil and Solvent-Resistant Nanofiltration. Green Chem. 2022, 24, 5291–5303. [Google Scholar] [CrossRef]
- Park, S.H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic Thin Film Composite Nanofiltration Membranes Derived Solely from Sustainable Sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Oviedo, C.; Szekely, G. Controlling the Degree of Acetylation in Cellulose-Based Nanofiltration Membranes for Enhanced Solvent Resistance. J. Membr. Sci. 2023, 687, 122040. [Google Scholar] [CrossRef]
- Zheng, D.; Hua, D.; Hong, Y.; Ibrahim, A.R.; Yao, A.; Pan, J.; Zhan, G. Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes 2020, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Falca, G.; Musteata, V.E.; Chisca, S.; Hedhili, M.N.; Ong, C.; Nunes, S.P. Naturally Extracted Hydrophobic Solvent and Self-Assembly in Interfacial Polymerization. ACS Appl. Mater. Interfaces 2021, 13, 44824–44832. [Google Scholar] [CrossRef] [PubMed]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and Further Expanding GSK’s Solvent Sustainability Guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the Next Generation of Sustainable Membranes from Green Chemistry Principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Pérez-Manríquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.V. Effective Interfacially Polymerized Polyester Solvent Resistant Nanofiltration Membrane from Bioderived Materials. Adv. Sustain. Syst. 2018, 2, 1800043. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Pérez-Manríquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.V. A Catechin/Cellulose Composite Membrane for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 567, 139–145. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Izzati Ayob, N.A.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Alammar, A.; Hardian, R.; Szekely, G. Upcycling Agricultural Waste into Membranes: From Date Seed Biomass to Oil and Solvent-Resistant Nanofiltration. Green Chem. 2022, 24, 365–374. [Google Scholar] [CrossRef]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of Sustainable Organic Solvent Nanofiltration Membranes Using Cellulose–Chitosan Biopolymer Blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Aburabie, J.H.; Puspasari, T.; Peinemann, K.V. Alginate-Based Membranes: Paving the Way for Green Organic Solvent Nanofiltration. J. Membr. Sci. 2020, 596, 117615. [Google Scholar] [CrossRef]
- George, N.; Varghese, V.; Kavitha, O. Earth’s versatile gift: Cellulose. In Handbook of Biomass; Springer: Singapore, 2023. [Google Scholar]
- Durmaz, E.N.; Zeynep Çulfaz-Emecen, P. Cellulose-Based Membranes via Phase Inversion Using [EMIM]OAc-DMSO Mixtures as Solvent. Chem. Eng. Sci. 2018, 178, 93–103. [Google Scholar] [CrossRef]
- Sukma, F.M.; Çulfaz-Emecen, P.Z. Cellulose Membranes for Organic Solvent Nanofiltration. J. Membr. Sci. 2018, 545, 329–336. [Google Scholar] [CrossRef]
- Konca, K.; Çulfaz-Emecen, P.Z. Effect of Carboxylic Acid Crosslinking of Cellulose Membranes on Nanofiltration Performance in Ethanol and Dimethylsulfoxide. J. Membr. Sci. 2019, 587, 117175. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic Liquids: Promising Compounds for Sustainable Chemical Processes and Applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Qiu, W.Z.; Zhong, Q.Z.; Du, Y.; Lv, Y.; Xu, Z.K. Enzyme-Triggered Coatings of Tea Catechins/Chitosan for Nanofiltration Membranes with High Performance. Green Chem. 2016, 18, 6205–6208. [Google Scholar] [CrossRef]
- Biron, M. A Practical Guide to Plastics Sustainability. In Concept, Solutions, and Implementation; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(Ethylene Terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Hardian, R.; Ghaffar, A.; Shi, C.; Chen, E.Y.-X.; Szekely, G. Chemically recyclable nanofiltration membranes fabricated from two circular polymer classes of the same monomer origin. J. Membr. Sci. 2024, 4, 100067. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/SA/en/products (accessed on 15 December 2023).
- Available online: https://www.thermofisher.com/it/en/home.html (accessed on 15 December 2023).
- Pérez-Manríquez, L.; Neelakanda, P.; Peinemann, K.V. Tannin-Based Thin-Film Composite Membranes for Solvent Nanofiltration. J. Membr. Sci. 2017, 541, 137–142. [Google Scholar] [CrossRef]
- Pérez-Manríquez, L.; Neelakanda, P.; Peinemann, K.V. Morin-Based Nanofiltration Membranes for Organic Solvent Separation Processes. J. Membr. Sci. 2018, 554, 1–5. [Google Scholar] [CrossRef]
- Liu, J.; Hua, D.; Zhang, Y.; Japip, S.; Chung, T.S. Precise Molecular Sieving Architectures with Janus Pathways for Both Polar and Nonpolar Molecules. Adv. Mater. 2018, 30, 1705933. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cook, M.; Park, S.H.; Moon, S.J.; Kim, J.F.; Livingston, A.G.; Lee, Y.M. A Compact and Scalable Fabrication Method for Robust Thin Film Composite Membranes. Green Chem. 2018, 20, 1887–1898. [Google Scholar] [CrossRef]
- Dedvukaj, A.; Raemdonck, S.; Vankelecom, I.F.J. Spray Coating as a Novel, Versatile Tool to Prepare Membranes via IP. J. Membr. Sci. 2023, 685, 121905. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; Shao, L.; Lau, C.H. Spray-Assisted Assembly of Thin-Film Composite Membranes in One Process. Adv. Membr. 2024, 4, 100080. [Google Scholar] [CrossRef]
- Gould, R.M.; White, L.S.; Wildemuth, C.R. Membrane Separation in Solvent Lube Dewaxing. Environ. Prog. 2001, 20, 12–16. [Google Scholar] [CrossRef]
- Lively, R.P.; Sholl, D.S. From Water to Organics in Membrane Separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Hu, J.; Kim, C.; Halasz, P.; Kim, J.F.; Kim, J.; Szekely, G. Artificial Intelligence for Performance Prediction of Organic Solvent Nanofiltration Membranes. J. Membr. Sci. 2021, 619, 118513. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Blanford, C.F.; Szekely, G. Reporting the Unreported: The Reliability and Comparability of the Literature on Organic Solvent Nanofiltration. Green Chem. 2020, 22, 3397–3409. [Google Scholar] [CrossRef]
- Ignacz, G.; Beke, A.K.; Szekely, G. Data-Driven Future for Nanofiltration: Escaping Linearity. J. Membr. Sci. Lett. 2023, 3, 100040. [Google Scholar] [CrossRef]
















| Driving Force | Pressure Difference (DP) | Osmotic Pressure Difference (Δπ) [Solvent Chemical Potential Gradient (Δμ)] | Concentration Difference (ΔC) | Temperature Difference (ΔT) | Electrical Potential Difference (Δf) |
|---|---|---|---|---|---|
| Phenomenological equation | Darcy’s law | Fick’s law | Fick’s law | Fourier’s law | Ohm’s law |
| Membrane operations | MF | FO | GS | MD | ED |
| UF | PV | EO | |||
| NF | D | ||||
| OSN | OSFO | ||||
| OSRO | |||||
| RO | RO |
| Method | Advantage | Disadvantages |
|---|---|---|
| Single-layer phase inversion |
|
|
| Dual-layer phase inversion |
|
|
| Interfacial Polymerization (TFC) |
|
|
| Incorporating fillers (MMM, TFN) |
|
|
| Post-treatment methods | ||
| Crosslinking |
|
|
| Solvent activation and Acid/alkali doping |
|
|
| Thermal annealing |
|
|
| Selective Layer | Support Layer | Membrane Active Area (cm2) | Membrane Type | Filtration Type | Solute | Solute MW (g mol−1) | Rejection (%) | Solvent | Permeance (L m−2 h−1 bar−1) | Pressure (bar) | Operation (days) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PANi | PANi | ~79 | Single layer | - | Oligostyrene | 400 | ~93 | Acetone | 1.50 | 6 | Screening | [105] |
| PEI/IPD | PP | 9 | TFC | Inside-out | SO BBR | 351 826 | 43 88 | Methanol | 1.47 | 4.13 | Screening | [32] |
| PBI | Crosslinked PI | 50 | Dual layer | Outside-in | MB | 319.85 | 99.17 99.55 | Methanol Acetonitrile | 2.60 1.58 | 1 | Screening | [78] |
| MPD–TMC | Crosslinked dual layer PI | ~45 | TFC | Outside-in | RBB | 626.54 | 99.3 | Methanol | 0.90 | 16 | Screening | [57] |
| PMDA–ODA PI/TiO2 | PMDA-ODA PI/TiO2 | 20 | MMM | Outside-in | FG | 808 | 90 | DMF | 2.5 | 10 | ~2 | [115] |
| PAI | PAI | ~16 | Single layer | Outside-in | RB | 1017.64 | 97.3 98.6 | Isopropanol DMF | 6.4 0.9 | 2 | 7 3 | [101] |
| PAN | PAN | 19 | Single layer | Outside-in | RBB | 626.54 | 99.9 | Ethanol | 2.32 | 2.8 | Screening | [102] |
| PI/NH2–MWCNTs | PI | - | MMM | Outside-in | MB BBR RBB TC | 319.85 826 626.54 444.4 | 99.8 99.9 99.9 97.4 | Isopropanol Acetone Methanol Ethanol | 0.53 4.31 2.26 1.17 | 5 | 2.5 | [80] |
| Polyamide 6 | Polyamide 6 | 2.59 | Single layer | Inside-out | Vitamin B12 | 1355 | 98 | Methanol | 0.21 | 3 | Screening | [116] |
| Cellulose | Cellulose | - | Single layer | Outside-in | CR BBR | 696 826 | >90 ~40 | Ethanol | 6 | 0.2 | 1 | [117] |
| MPD–TMC/TiO2@rGO | Si3N4/PAN | 22 | TFN | Outside-in | BTB RB | 624 1017.64 | 95 98 | Ethanol | ~4.6 ~3.3 | 8 | Screening | [62] |
| PIM–1/AlOx | Crosslinked PI | - | TFN | Outside-in | RB | 1017.64 | 86 | Ethanol | ~1.5 | 0.69 | Screening | [118] |
| PBI | PBI | 20.10 | Single layer | Outside-in | MB RB | 319.85 1017.64 | ~99 ~99 | Acetone Methanol | ~4 ~1 | 5 | Screening 1 | [46] |
| GO | Crosslinked polyetherimide | 4.73 | TFN | Outside-in | RB | 1017.64 | 91 | Acetone | 4 | 1 | Screening | [94] |
| PI/FTB | Crosslinked polyetherimide | - | Dual layer | Outside-in | TC | 444.4 | >99 | Methanol | 3.7 | - | Screening | [46] |
| PEI/PIP–TMC | Crosslinked PI | ~32 | TFC | Inside-out | AF RB | 585 1017.64 | 90.3 99.9 | Acetone Isopropanol | 10.8 4.5 | 2 | 3 Screening | [58] |
| PAN | PAN | 23 | Single layer | Outside-in | EB MB | 960.81 319.85 | 100 71.5 | Methanol | 1.28 | 3 | 7 Screening | [113] |
| PI | PI | - | Single layer | Outside-in | RDB | 479 | 96.5 | Ethanol | 0.45 | 10 | Screening | [119] |
| PI | Modified PET braids with alkaline treatment | 2000 | Composite | Outside-in | TC | 444.4 | 99.2 | DMF Methanol | 2.03 4.94 | 6 | Screening | [71] |
| MPD–TMC | Crosslinked PI | 724 | TFC | Inside-out | MR MR Levofloxacin MO AF RB | 269 269 361 327 585 1017.84 | 90 90.1 98.2 98.6 99.4 99.8 | Acetone | 23.5 24.2 | 2 | 7 Screening | [59] |
| Polyamide 6 | Polyamide 6 | 60 | MMM | Inside-out | Vitamin B12 | 1355 | >99 | Methanol | 0.1 | 8 | Screening | [120] |
| PAI | PAI | ~9 | Single layer | Outside-in | MB VbB | 319.85 506.1 | 99.7 99.9 | Methanol | 1 1.87 | 2 | Screening | [121] |
| PBI | PBI | 2200 | Single layer | Outside-in | MO RBB TG | 327.33 626.54 885 | 77.5 99.5 99 | Acetone | 1.85 1.95 0.35 | 10 | Screening 30 | [104] |
| PI | PI | - | Single layer | Outside-in | RDB | 479.01 | 98 | Ethanol | 1.83 | 10 | Screening | [70] |
| PPy | Braid reinforced PPTA | ~25 | Composite | Outside-in | MB Eosin Y CR | 319.85 647.89 696.7 | 84.5 93.4 99.3 99.5 | DMAc Ethanol | 1.04 1.10 1.17 1.64 | 6 | Screening | [112] |
| MPD–TMC/GO | Crosslinked PI | 0.17 | TFC | Outside-in | RDB RB | 479.01 1017.84 | ~100 99 | Ethanol Methanol | 1.80 5.80 | 5 | Screening | [59] |
| BTCA–TAPA/COF | Crosslinked PI | 0.44 | TFC | Inside-out | L-α-lecithin FG RB | 758 808 1017.84 | ~100 98.9 99.5 ~99 92 99.1 | Hexane Acetone Isopropanol Ethanol Isopropanol | 266.27 395.21 61.68 ~50 98.44 61.68 | 1 | Screening 7.29 Screening | [61] |
| PBI | PET | 544 | Composite | Outside-in | RB | 1017.84 | 99.5 | Methanol | 3.60 1.37 | 10 | Screening ~3 | [48] |
| PEI/SMA | PVDF | - | Composite | Outside-in | MO CV | 327.3 407 | 99.9 ~98 | Ethanol | 6.4 1.90 0.70 | 1 | Screening 1 | [63] |
| PEI–TMC PEI/PIP–TMC | PTFE modified with PDA coating | 6 | TFC | Inside-out | AF MO | 585 327.3 | 94.8 93 99.9 95.4 | DMF DMF | 3.70 2.44 1.79 | 2 | Screening 3 Screening | [64] |
| Selective Layer | Support Layer | Membrane Active Area (cm2) | Solute | Solute MW (g mol−1) | Rejection (%) | Solvent | Permeance (L m−2 h−1 bar−1) | Pressure (bar) | Operation (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan and FDA | Recycled PET | 52 | MO RB | 327.33 1017.64 | 91.6 ~100 | Acetone | 11.9 | 10 | 14 | [136] |
| Priamine–genipin | PLA with gelatin as the interlayer layer | 28 | Oleuropein AF | 540.51 585.54 | 99 100 | Acetone | 9.9 | 30 | 7 | [138] |
| Priamine–TA | Recycled PET | 52 | Methyl styrene dimer | 235 | ~90 ~93 ~75 | Acetone Heptane Toluene | 13.5 ~2.5 ~3.5 | - | 7 | [139] |
| Quercetin–TPC | Cellulose | 12.6 | SO MO TB AB BB Reactive black Reactive green | 248.28 327.33 466.59 595.50 792.84 991.80 1418.93 | 58 90 94 96 97 98 100 | DMF | 2.6 2.4 2.7 2.6 2.4 2.6 2.8 | 10 | Screening | [146] |
| TA–TPC | Crosslinked PAN | 12.6 | CR BB RB | 696.66 825.97 1017.64 | 88 95 93 | NMP | 0.09 0.08 0.08 | 20 | Screening | [165] |
| Morin hydrate–TPC | Crosslinked PAN | 12.6 | Solvent blue 35 Polystyrene CR RBB BB RB | 350 500 590.71 626 825.97 1017.64 | 62 93 89 94 96 97 | NMP | 0.1 0.06 0.5 0.2 0.3 0.2 | 20 | Screening | [166] |
| Catechin–TPC | Cellulose | 12.6 | AB | 617 | 92 | DMF | 1.4 | 10 | Screening | [147] |
| Cyclodextrin–TMC | Crosslinked PI | - | RBB | 626 | 98.8 | Ethanol | 2.9 | 10 | Screening | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alammar, A.Y.; Choi, S.-H.; Buonomenna, M.G. Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration. Processes 2024, 12, 563. https://doi.org/10.3390/pr12030563
Alammar AY, Choi S-H, Buonomenna MG. Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration. Processes. 2024; 12(3):563. https://doi.org/10.3390/pr12030563
Chicago/Turabian StyleAlammar, Abdulaziz Y., Seung-Hak Choi, and Maria Giovanna Buonomenna. 2024. "Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration" Processes 12, no. 3: 563. https://doi.org/10.3390/pr12030563






