Mathematical Model of Graphene Yield in Ultrasonic Preparation
Abstract
:1. Introduction
2. Experimental Materials, Characterization and Modeling Methods
2.1. Materials and Experiments
2.2. Analysis and Characterization
2.2.1. Concentration Determination and Yield of Graphene Dispersions
2.2.2. Scanning Electron Microscope
2.2.3. Raman Spectra
2.2.4. Transmission Electron Microscopy
2.2.5. Atomic Force Microscopy
2.3. Modeling Methods
3. Experimental Results and Analysis
3.1. Establishment of Regression Model
3.2. Effect of Ultrasound Process Parameters on Graphene Yield
4. Experimental Verification
4.1. Characterization of Graphene Prepared by Ultrasonic Exfoliation Method
4.2. Raman Spectral Analysis
4.3. Transmission Electron Microscopy and Atomic Force Microscopy Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Deng, N.; Li, J.; He, G.; Li, J. Highly Thermal-Conductive Graphite Flake/Cu Composites Prepared by Sintering Intermittently Electroplated Core-Shell Powders. J. Mater. Sci. Technol. 2021, 61, 93–99. [Google Scholar] [CrossRef]
- Dai, W.; Lv, L.; Ma, T.; Wang, X.; Ying, J.; Yan, Q.; Tan, X.; Gao, J.; Xue, C.; Yu, J.; et al. Multiscale Structural Modulation of Anisotropic Graphene Framework for Polymer Composites Achieving Highly Efficient Thermal Energy Management. Adv. Sci. 2021, 8, 2003734. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene Growth on Silicon Carbide: A Review. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Chen, S. Large Area CVD Growth of Graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Bahri, M.; Gebre, S.H.; Elaguech, M.A.; Dajan, F.T.; Sendeku, M.G.; Tlili, C.; Wang, D. Recent Advances in Chemical Vapour Deposition Techniques for Graphene-Based Nanoarchitectures: From Synthesis to Contemporary Applications. Coord. Chem. Rev. 2023, 475, 214910. [Google Scholar] [CrossRef]
- Navik, R.; Gai, Y.; Wang, W.; Zhao, Y. Curcumin-Assisted Ultrasound Exfoliation of Graphite to Graphene in Ethanol. Ultrason. Sonochem. 2018, 48, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hu, G.; Gao, H. Preparation of Few-Layer and Single-Layer Graphene by Exfoliation of Expandable Graphite in Supercritical N,N-Dimethylformamide. J. Supercrit. Fluids 2012, 63, 99–104. [Google Scholar] [CrossRef]
- Oduncu, M.R.; Ke, Z.; Zhao, B.; Shang, Z.; Simpson, R.; Wang, H.; Wei, A. Exfoliation and Spray Deposition of Graphene Nanoplatelets in Ethyl Acetate and Acetone: Implications for Additive Manufacturing of Low-Cost Electrodes and Heat Sinks. ACS Appl. Nano Mater. 2023, 6, 14574–14582. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Wang, Q.B.; Yin, J.Z. High-Pressure Induced Exfoliation for Regulating the Morphology of Graphene in Supercritical CO2 System. Carbon 2021, 178, 211–222. [Google Scholar] [CrossRef]
- Morton, J.A.; Kaur, A.; Khavari, M.; Tyurnina, A.V.; Priyadarshi, A.; Eskin, D.G.; Mi, J.; Porfyrakis, K.; Prentice, P.; Tzanakis, I. An Eco-Friendly Solution for Liquid Phase Exfoliation of Graphite under Optimised Ultrasonication Conditions. Carbon 2023, 204, 434–446. [Google Scholar] [CrossRef]
- Morton, J.A.; Khavari, M.; Priyadarshi, A.; Kaur, A.; Grobert, N.; Mi, J.; Porfyrakis, K.; Prentice, P.; Eskin, D.G.; Tzanakis, I. Dual Frequency Ultrasonic Cavitation in Various Liquids: High-Speed Imaging and Acoustic Pressure Measurements. Phys. Fluids 2023, 35, 017135. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Varrla, E.; Paton, K.R.; Backes, C.; Harvey, A.; Smith, R.J.; McCauley, J.; Coleman, J.N. Turbulence-Assisted Shear Exfoliation of Graphene Using Household Detergent and a Kitchen Blender. Nanoscale 2014, 6, 11810–11819. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W.S.W. Ultrasonication an Intensifying Tool for Preparation of Stable Nanofluids and Study the Time Influence on Distinct Properties of Graphene Nanofluids—A Systematic Overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic Exfoliation of Graphene in Water: A Key Parameter Study. Carbon 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Hadi, A.; Zahirifar, J.; Karimi-Sabet, J.; Dastbaz, A. Graphene Nanosheets Preparation Using Magnetic Nanoparticle Assisted Liquid Phase Exfoliation of Graphite: The Coupled Effect of Ultrasound and Wedging Nanoparticles. Ultrason. Sonochem. 2018, 44, 204–214. [Google Scholar] [CrossRef]
- Morton, J.A.; Eskin, D.G.; Grobert, N.; Mi, J.; Porfyrakis, K.; Prentice, P.; Tzanakis, I. Effect of Temperature and Acoustic Pressure During Ultrasound Liquid-Phase Processing of Graphite in Water. JOM 2021, 73, 3745–3752. [Google Scholar] [CrossRef]
- Kaur, A.; Morton, J.A.; Tyurnina, A.V.; Priyadarshi, A.; Holland, A.; Mi, J.; Porfyrakis, K.; Eskin, D.G.; Tzanakis, I. Temperature as a Key Parameter for Graphene Sono-Exfoliation in Water. Ultrason. Sonochem. 2022, 90, 106187. [Google Scholar] [CrossRef] [PubMed]
- Tyurnina, A.V.; Morton, J.A.; Subroto, T.; Khavari, M.; Maciejewska, B.; Mi, J.; Grobert, N.; Porfyrakis, K.; Tzanakis, I.; Eskin, D.G. Environment Friendly Dual-Frequency Ultrasonic Exfoliation of Few-Layer Graphene. Carbon 2021, 185, 536–545. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Morton, J.A.; Kaur, A.; Mi, J.; Grobert, N.; Porfyrakis, K.; Tzanakis, I.; Eskin, D.G. Effects of Green Solvents and Surfactants on the Characteristics of Few-Layer Graphene Produced by Dual-Frequency Ultrasonic Liquid Phase Exfoliation Technique. Carbon 2023, 206, 7–15. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, W.; Wang, W.; Wang, Y.; Zhao, Y.; Lei, Z.; Miao, R. Ultrasonic-Assisted Production of Graphene with High Yield in Supercritical CO2 and Its High Electrical Conductivity Film. Ind. Eng. Chem. Res. 2014, 53, 2839–2845. [Google Scholar] [CrossRef]
- Navik, R.; Tan, H.; Liu, Z.; Xiang, Q.; Zhao, Y. Scalable Production of High-Quality Exfoliated Graphene Using Mechanical Milling in Conjugation with Supercritical CO2. FlatChem 2022, 33, 100374. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Jia, J.; Wang, W.; Gao, Y.; Zhao, Y.; Chen, Y. Green Production of Pristine Graphene Using Fluid Dynamic Force in Supercritical CO2. Chem. Eng. J. 2016, 298, 198–205. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wu, Z.; Li, Y.; Yang, W.; Li, Y. High-Efficiency Production of Graphene by Supercritical CO2 Exfoliation with Rapid Expansion. Langmuir 2018, 34, 7797–7804. [Google Scholar] [CrossRef]
- Adel, M.; Abdel-Karim, R.; Abdelmoneim, A. Studying the Conversion of Graphite into Nanographene Sheets Using Supercritical Phase Exfoliation Method. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 589–602. [Google Scholar] [CrossRef]
- Szroeder, P.; Górska, A.; Tsierkezos, N.; Ritter, U.; Strupiński, W. The Role of Band Structure in Electron Transfer Kinetics in Low-Dimensional Carbon. Materialwiss. Werkstofftech. 2013, 44, 226–230. [Google Scholar] [CrossRef]
- Hadi, A.; Karimi-Sabet, J.; Moosavian, S.M.A.; Ghorbanian, S. Optimization of Graphene Production by Exfoliation of Graphite in Supercritical Ethanol: A Response Surface Methodology Approach. J. Supercrit. Fluids 2016, 107, 92–105. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical Functionalization of Graphene and Its Applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]









| Dilution Factor | Absorbance | Calculated Concentration (mg/mL) | Actual Concentration (mg/mL) |
|---|---|---|---|
| 50 times | 0.270 | 0.106 | 0.1 |
| 0.272 | 0.107 | ||
| 0.272 | 0.107 | ||
| 60 times | 0.212 | 0.083 | 0.083 |
| 0.214 | 0.084 | ||
| 0.212 | 0.083 | ||
| 100 times | 0.132 | 0.051 | 0.05 |
| 0.130 | 0.050 | ||
| 0.130 | 0.050 |
| Number | Factor Name | Horizontal Code | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| x1 | Ultrasonication time/min | 30 | 90 | 150 |
| x2 | Ultrasonic power/W | 300 | 900 | 1500 |
| x3 | Graphite initial weight/g | 0.5 | 1 | 1.5 |
| Group | Ultrasonication Time/min | Ultrasonic Power/W | Graphite Initial Weight/g | Concentration (mg/mL) | Yield |
|---|---|---|---|---|---|
| 1 | 30 | 300 | 1 | 0.51 | 0.102 |
| 2 | 90 | 300 | 0.5 | 0.46 | 0.184 |
| 3 | 90 | 300 | 1.5 | 0.43 | 0.057 |
| 4 | 150 | 300 | 1 | 0.11 | 0.022 |
| 5 | 30 | 900 | 0.5 | 0.78 | 0.312 |
| 6 | 30 | 900 | 1.5 | 0.82 | 0.109 |
| 7 | 90 | 900 | 1 | 0.41 | 0.082 |
| 8 | 90 | 900 | 1 | 0.56 | 0.112 |
| 9 | 90 | 900 | 1 | 0.57 | 0.114 |
| 10 | 150 | 900 | 0.5 | 0.33 | 0.132 |
| 11 | 150 | 900 | 1.5 | 0.24 | 0.032 |
| 12 | 30 | 1500 | 1 | 0.84 | 0.168 |
| 13 | 90 | 1500 | 0.5 | 0.56 | 0.224 |
| 14 | 90 | 1500 | 1.5 | 0.46 | 0.061 |
| 15 | 150 | 1500 | 1 | 0.21 | 0.042 |
| Model | Projects and Factors | Sum of Squares | df | Mean Square | F Value | p Value Prob > F | Significance |
|---|---|---|---|---|---|---|---|
| Graphene yield | Model | 0.086 | 8 | 0.011 | 52.38 | <0.0001 | Significant |
| x1 | 0.027 | 1 | 0.027 | 130.99 | <0.0001 | ||
| x2 | 0.002 | 1 | 2.1 × 10−3 | 10.33 | 0.0183 | ||
| x3 | 0.044 | 1 | 0.044 | 214.87 | <0.0001 | ||
| x1 × x2 | 5.29 × 10−4 | 1 | 5.29 × 10−4 | 2.59 | 0.1589 | ||
| x1 × x3 | 2.65 × 10−3 | 1 | 2.65 × 10−3 | 12.96 | 0.0114 | ||
| x2 × x3 | 3.24 × 10−4 | 1 | 3.24 × 10−4 | 1.58 | 0.2550 | ||
| x22 | 1.05 × 10−3 | 1 | 1.05 × 10−3 | 5.12 | 0.0644 | ||
| x32 | 7.85 × 10−3 | 1 | 7.85 × 10−3 | 38.35 | 0.0008 | ||
| Lack of fit | 6.7 × 10−4 | 4 | 1.46 × 10−4 | 0.45 | 0.7730 | Not Significant |
| Process Parameters | Average Concentration/(mg/mL) | Yield |
|---|---|---|
| 1500 W and ultrasonication time 20 min | 0.639 | 25.6% |
| 1500 W and ultrasonication time 30 min | 0.790 | 31.6% |
| 1500 W and ultrasonication time 40 min | 0.715 | 28.6% |
| 1500 W and ultrasonication time 60 min | 0.621 | 24.8% |
| 900 W and ultrasonication time 30 min | 0.780 | 31.2% |
| 900 W and ultrasonication time 60 min | 0.599 | 24% |
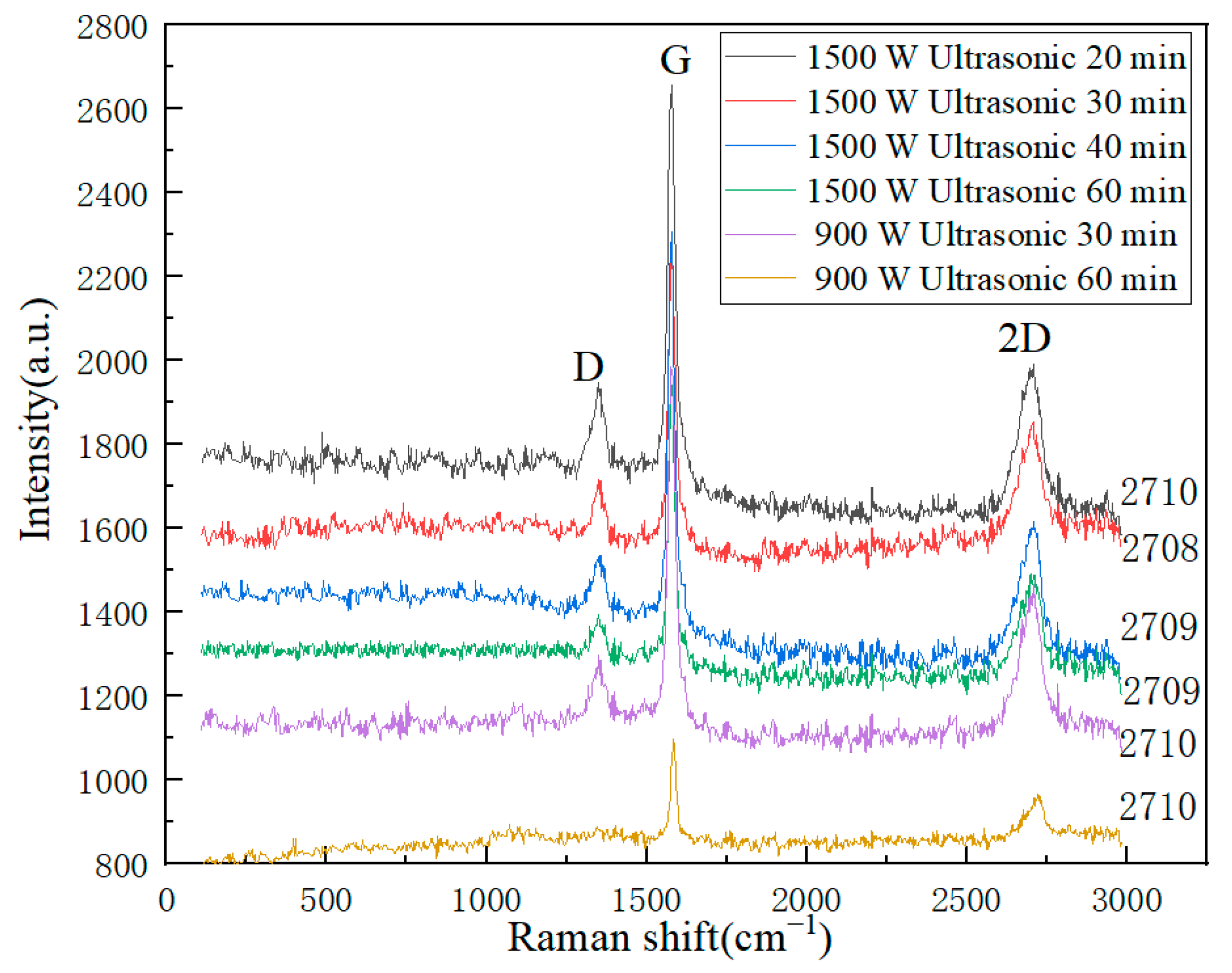
| Process Parameters | Position (cm−1) | Average Value | Average Value | ||
|---|---|---|---|---|---|
| D | G | 2D | ID/IG | I2D/IG | |
| 1500 W and ultrasonication time 20 min | ~1350 | ~1579 | ~2710 | 0.73 | 0.75 |
| 1500 W and ultrasonication time 30 min | ~1350 | ~1578 | ~2709 | 0.77 | 0.83 |
| 1500 W and ultrasonication time 40 min | ~1351 | ~1579 | ~2709 | 0.66 | 0.71 |
| 1500 W and ultrasonication time 60 min | ~1350 | ~1577 | ~2709 | 0.73 | 0.77 |
| 900 W and ultrasonication time 30 min | ~1350 | ~1576 | ~2710 | 0.70 | 0.77 |
| 900 W and ultrasonication time 60 min | ~1351 | ~1576 | ~2710 | 0.72 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Gu, B.; Kan, C.; Lv, X.; Wang, Z.; Yang, P.; Zhao, H. Mathematical Model of Graphene Yield in Ultrasonic Preparation. Processes 2024, 12, 674. https://doi.org/10.3390/pr12040674
Yi J, Gu B, Kan C, Lv X, Wang Z, Yang P, Zhao H. Mathematical Model of Graphene Yield in Ultrasonic Preparation. Processes. 2024; 12(4):674. https://doi.org/10.3390/pr12040674
Chicago/Turabian StyleYi, Jinquan, Baoshan Gu, Chengling Kan, Xudong Lv, Zhifeng Wang, Peiyan Yang, and Haoqi Zhao. 2024. "Mathematical Model of Graphene Yield in Ultrasonic Preparation" Processes 12, no. 4: 674. https://doi.org/10.3390/pr12040674






