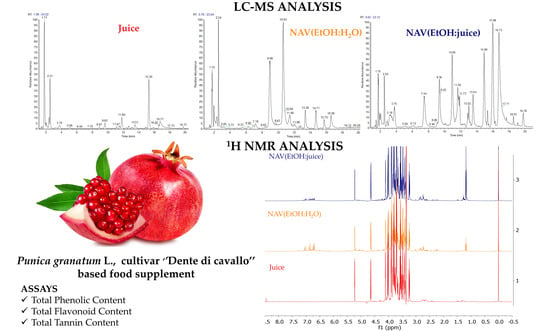
Development of a New Extraction Method for Pomegranate and Metabolite Profiling by a LC-MS and 1H NMR Combined Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation and Extraction Procedure
2.3. Analysis by LC-ESI/QExactive/MS/MS
2.4. 1H-NMR Analysis and Data Processing
2.5. Determination of Total Phenolic and Total Tannin Content
2.6. Determination of Total Flavonoid Content
3. Results and Discussion
3.1. Extraction Procedure of Pomegranate
3.2. LC-ESI/QExactive/MS/MS Analysis
3.3. 1H-NMR Analysis of P. granatum Juice and Extracts
3.4. Total Phenolic, Tannin and Flavonoid Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food. Chem. 2022, 395, 133600. [Google Scholar]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar]
- Borochov-Neori, H.; Judeinstein, S.; Tripler, E.; Harari, M.; Greenberg, A.; Shomer, I.; Holland, D. Seasonal and cultivar variations in antioxidant and sensory quality of pomegranate (Punica granatum L.) fruit. J. Food Compos. Anal. 2009, 22, 189–195. [Google Scholar] [CrossRef]
- Cruz-Valenzuela, M.R.; Ayala-Soto, R.E.; Ayala-Zavala, J.F.; Espinoza-Silva, B.A.; González-Aguilar, G.A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Nazzaro, F.; Fratianni, F.; Tapia-Rodríguez, M.R.; et al. Pomegranate (Punica granatum L.) Peel Extracts as Antimicrobial and Antioxidant Additives Used in Alfalfa Sprouts. Foods 2022, 11, 2588. [Google Scholar]
- Toledo-Merma, P.R.; Cornejo-Figueroa, M.H.; Crisosto-Fuster, A.D.R.; Strieder, M.M.; Chañi-Paucar, L.O.; Náthia-Neves, G.; Rodríguez-Papuico, H.; Rostagno, M.A.; Meireles, M.A.A.; Alcázar-Alay, S.C. Phenolic Compounds Recovery from Pomegranate (Punica granatum L.) By-Products of Pressurized Liquid Extraction. Foods 2022, 11, 1070. [Google Scholar]
- Maghoumi, M.; Amodio, M.L.; Fatchurrahman, D.; Cisneros-Zevallos, L.; Colelli, G. Pomegranate Husk Scald Browning during Storage: A Review on Factors Involved, Their Modes of Action, and Its Association to Postharvest Treatments. Foods 2022, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M.; et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappaB-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Guo, Y.; Wang, T.; Pantuck, A.J.; Rettig, M.B. Pomegranate extract inhibits EMT in clear cell renal cell carcinoma in a NF-κB and JNK dependent manner. Asian J. Urol. 2015, 2, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellstrom, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit alpha-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef]
- Rodriguez, J.; Gilson, H.; Jamart, C.; Naslain, D.; Pierre, N.; Deldicque, L.; Francaux, M. Pomegranate and green tea extracts protect against ER stress induced by a high-fat diet in skeletal muscle of mice. Eur. J. Nutr. 2015, 54, 377–389. [Google Scholar] [CrossRef]
- Middha, S.K.; Usha, T.; RaviKiran, T. Influence of Punica granatum L. on region specific responses in rat brain during Alloxan-Induced diabetes. Asian Pac. J. Trop. Biomed. 2012, 2, S905–S909. [Google Scholar]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Singh, R.P. Studies on Antioxidant Activity of Pomegranate (Punica granatum) Peel Extract Using in Vivo Models. J. Agric. Food Chem. 2002, 50, 4791–4795. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Bugert, J.; Denyer, S.P.; Heard, C.M. Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017, 112, 30–37. [Google Scholar]
- Hartman, R.E.; Shah, A.; Fagan, A.M.; Schwetye, K.E.; Parsadanian, M.; Schulman, R.N.; Finn, M.B.; Holtzman, D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006, 24, 506–515. [Google Scholar] [CrossRef]
- Ropacki, S.A.; Patel, S.M.; Hartman, R.E. Pomegranate Supplementation Protects against Memory Dysfunction after Heart Surgery: A Pilot Study. Evid.-Based Complement.Alternat. Med. 2013, 2013, 932401. [Google Scholar]
- Gadkari, P.; Daharwal, S.J. Quantification of Punicalagin in Pomegranate Peels from High-performance Thin-layer Chromatography. Biomed. Biotechnol. Res. J. 2022, 6, 586–590. [Google Scholar] [CrossRef]
- Abdul Hamid, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91. [Google Scholar] [CrossRef]
- Marra, F.; Petrovicova, B.; Canino, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Pomegranate Wastes Are Rich in Bioactive Compounds with Potential Benefit on Human Health. Molecules 2022, 27, 5555. [Google Scholar] [CrossRef]
- Arlotta, C.; Toscano, V.; Genovese, C.; Calderaro, P.; Puglia, G.D.; Raccuia, S.A. Nutraceutical Content and Genetic Diversity Share a Common Pattern in New Pomegranate Genotypes. Molecules 2022, 27, 389. [Google Scholar] [CrossRef]
- Tarantino, A.; Difonzo, G.; Disciglio, G.; Frabboni, L.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Fresh pomegranate juices from cultivars and local ecotypes grown in southeastern Italy: Comparison of physicochemical properties, antioxidant activity and bioactive compounds. J. Sci. Food Agric. 2022, 102, 1185–1192. [Google Scholar] [CrossRef]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, Antiglycation, and Cytoprotective Properties of a Phenol-Rich Extract From Waste Peel of var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef]
- Deborde, C.; Moing, A.; Roch, L.; Jacob, D.; Rolin, D.; Giraudeau, P. Plant metabolism as studied by NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102–103, 61–97. [Google Scholar]
- Masullo, M.; Cerulli, A.; Mari, A.; de Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food. Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Piacente, S. Metabolite Profiling of Helichrysum italicum Derived Food Supplements by H-1-NMR-Based Metabolomics. Molecules 2021, 26, 6619. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić Martinović, L.; Malenica, M.; Gobin, I.; Pedisić, S.; Dragović-Uzelac, V.; Kraljević Pavelić, S. Assessment of the Biological Activity and Phenolic Composition of Ethanol Extracts of Pomegranate (Punica granatum L.) Peels. Molecules 2020, 25, 5916. [Google Scholar]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods 2022, 11, 2596. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Parisi, V.; Santoro, V.; Donadio, G.; Bellone, M.L.; Diretto, G.; Sandri, C.; Mensitieri, F.; De Tommasi, N.; Dal Piaz, F.; Braca, A. Comparative Chemical Analysis of Eight Punica granatum L. Peel Cultivars and Their Antioxidant and Anti-Inflammatory Activities. Antioxidants 2022, 11, 2262. [Google Scholar]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut shells (Italian cultivar “Marrone di Roccadaspide” PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MSn rationalization of tannins. Food. Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Maldini, M.; Montoro, P.; Hamed, A.I.; Mahalel, U.A.; Oleszek, W.; Stochmal, A.; Piacente, S. Strong antioxidant phenolics from Acacia nilotica: Profiling by ESI-MS and qualitative-quantitative determination by LC-ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 228–239. [Google Scholar] [CrossRef]
- Akter, S.; Hong, H.; Netzel, M.; Tinggi, U.; Fletcher, M.; Osborne, S.; Sultanbawa, Y. Determination of Ellagic Acid, Punicalagin, and Castalagin from Terminalia ferdinandiana (Kakadu plum) by a Validated UHPLC-PDA-MS/MS Methodology. Food Anal. Methods 2021, 14, 2534–2544. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, K.W.; Wu, D.-T.; Liu, H.-Y.; Li, H.-B.; Zhang, J.-R.; Gan, R.-Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef]
- Abdulla, R.; Mansur, S.; Lai, H.; Ubul, A.; Sun, G.; Huang, G.; Aisa, H.A. Qualitative Analysis of Polyphenols in Macroporous Resin Pretreated Pomegranate Husk Extract by HPLC-QTOF-MS. Phytochem. Anal. 2017, 28, 465–473. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Hosek, J.; Pizza, C.; Piacente, S. Metabolite profiling of “green” extracts of Corylus avellana leaves by H-1 NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 160, 168–178. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite Profiling of “Green” Extracts of Cynara cardunculus subsp. scolymus, Cultivar “Carciofo di Paestum” PGI by 1H NMR and HRMS-Based Metabolomics. Molecules 2022, 27, 3328. [Google Scholar]
- Kraszni, M.; Marosi, A.; Larive, C.K. NMR assignments and the acid-base characterization of the pomegranate ellagitannin punicalagin in the acidic pH-range. Anal. Bioanal. Chem. 2013, 405, 5807–5816. [Google Scholar] [CrossRef]
- Cicero, N.; Corsaro, C.; Salvo, A.; Vasi, S.; Giofre, S.V.; Ferrantelli, V.; Di Stefano, V.; Mallamace, D.; Dugo, G. The metabolic profile of lemon juice by proton HR-MAS NMR: The case of the PGI Interdonato Lemon of Messina. Nat. Prod. Res. 2015, 29, 1894–1902. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Ariburnu, E.; Masullo, M.; Festa, M.; Capasso, A.; Yesilada, E.; Piacente, S. Iridoid, phenylethanoid and flavonoid glycosides from Sideritis trojana. Fitoterapia 2012, 83, 130–136. [Google Scholar] [CrossRef]
- Borim de Souza, A.J.; Ocampos, F.M.M.; Catoia Pulgrossi, R.; Dokkedal, A.L.; Colnago, L.A.; Cechin, I.; Saldanha, L.L. NMR-Based Metabolomics Reveals Effects of Water Stress in the Primary and Specialized Metabolisms of Bauhinia ungulata L. (Fabaceae). Metabolites 2023, 13, 381. [Google Scholar]
- Corol, D.I.; Harflett, C.; Beale, M.H.; Ward, J.L. An Efficient High Throughput Metabotyping Platform for Screening of Biomass Willows. Metabolites 2014, 4, 946–976. [Google Scholar] [CrossRef]
- Kılınc, H.; D’Urso, G.; Paolillo, A.; Alankus, O.; Piacente, S.; Masullo, M. LC-MS and NMR Based Plant Metabolomics: A Comprehensive Phytochemical Investigation of Symphytum anatolicum. Metabolites 2023, 13, 1051. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and Extraction Methods of Polyphenolic Compounds Derived from Pomegranate (Punica granatum) Peels. A Mini Review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.L.; Zhang, K.; Wan, Y.S.; Gao, X.H.; Chen, H.D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]




| Rt | [M-H]− | [M-H]2− | Molecular Formula | Δppm | MS/MS | Name | NAV (EtOH:juice) | NAV (EtOH:H2O) | Juice | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.50 | 481.0625 | C20H18O14 | 2.53 | 421.0427, 300.9991, 275.0199 | HHDP-D-glucose | √ | √ | √ | |
| 2 | 3.75 | 781.0542 | C34H22O22 | 2.31 | 600.9899, 575.0115, 300.9988 | Punicalin | √ | √ | ||
| 3 | 5.09 | 649.0697 | C27H22O19 | 2.48 | 498.5004, 300.9991 | Lagerstannin C | √ | √ | ||
| 4 | 7.44 | 305.0668 | C15H14O7 | 3.09 | 287.0563, 219.0659, 179.0343, 165.0185, 137.0234, 125.0233 | Epigallocatechin | √ | √ | ||
| 5 | 9.36 | 1083.0601 | 541.0262 | C48H28O30 | 2.37 | 600.9901, 575.0101, 300.9991, 275.0199 | Punicalagin isomer α | √ | √ | √ |
| 6 | 10.95 | 1083.0601 | 541.0262 | C48H28O30 | 1.81 | 600.9901, 575.0101, 300.9991, 275.0199 | Punicalagin isomer β | √ | √ | √ |
| 7 | 11.62 | 799.0646 | C34H24O23 | 2.64 | 300.9991, 273.0043 | Granatin A | √ | √ | √ | |
| 8 | 12.99 | 785.0857 | C34H26O22 | 3.17 | 300.9991, 275.0199, 249.0403 | Pedunculagin II | √ | √ | √ | |
| 9 | 13.02 | 633.0742 | C27H22O18 | 3.03 | 463.0528, 419.0617, 300.9991, 275.0198 | Galloyl-HHDP-hexoside | √ | √ | ||
| 10 | 13.54 | 463.0520 | C20H16O13 | 3.83 | 300.9991, 275.0199, 249.0403 | Ellagic acid glucoside | √ | √ | √ | |
| 11 | 14.89 | 951.0748 | C41H28O27 | 1.48 | 300.9991, 273.0043, | Granatin B | √ | √ | √ | |
| 12 | 15.32 | 475.1824 | C21H32O12 | 2.94 | 300.9984, 169.0133 | Kanokoside A | √ | √ | ||
| 13 | 15.98 | 433.0414 | C19H14O12 | 2.83 | 300.9987 | Ellagic acid pentose | √ | √ | ||
| 14 | 16.73 | 300.9991 | C14H6O8 | 3.54 | / | Ellagic acid | √ | √ | √ | |
| 15 | 17.71 | 593.1523 | C27H30O15 | 3.68 | 285.0404, 255.0298 | Kaempferol 3-O-rutinoside | √ | |||
| 16 | 18.55 | 447.0937 | C21H20O11 | 3.36 | 301.0003, 284.0327, 255.0298 | Astragalin | √ | √ | ||
| 17 | 19.78 | 447.0939 | C21H20O11 | 3.69 | 300.9984, 285.0405 | Luteolin 4’-O-glucoside | √ | √ |
| Metabolite | NAV (EtOH:juice) | NAV (EtOH:H2O) | Juice |
|---|---|---|---|
| Alanine | 0.096 ± 0.004 | 0.046 ± 0.004 | 0.047 ± 0.001 |
| Ascorbate | 0.309 ± 0.047 | 0.233 ± 0.043 | 0.137 ± 0.009 |
| Ellagic acid (14) | 0.129 ± 0.007 | 0.066 ± 0.005 | 0.003 ± 0.001 |
| Fructose | 17.018 ± 0.482 | 10.677 ± 0.623 | 20.477 ± 0.506 |
| Glucose | 14.233 ± 0.637 | 11.435 ± 0.623 | 16.994 ± 0.414 |
| Glutamine | 0.128 ± 0.029 | 0.123 ± 0.024 | 0.070 ± 0.010 |
| Malate | 0.443 ± 0.050 | 0.266 ± 0.038 | 0.322 ± 0.019 |
| Punicalagin isomers (5, 6) | 0.496 ± 0.036 | 1.133 ± 0.055 | 0.022 ± 0.004 |
| Threonine | 0.071 ± 0.013 | 0.028 ± 0.006 | 0.066 ± 0.006 |
| P. granatum | Total Phenol Content (mg GAE/g ± SD) a | Total Flavonoid Content (mg rutin/g ± SD) a | Total Tannin Content (mg GAE/g ± SD) a |
|---|---|---|---|
| Juice | 64.60 ± 2.52 | 268.17 ± 0.58 | 1.77 ± 1.02 |
| NAV(EtOH:H2O) | 114.10 ± 4.82 | 154.83 ± 0.25 | 97.03 ± 1.89 |
| NAV(EtOH:juice) | 484.27 ± 1.29 | 432.33 ± 1.59 | 71.47 ± 2.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polcaro, L.M.; Samani, M.R.; Piacente, S.; Masullo, M. Development of a New Extraction Method for Pomegranate and Metabolite Profiling by a LC-MS and 1H NMR Combined Approach. Foods 2024, 13, 1429. https://doi.org/10.3390/foods13101429
Polcaro LM, Samani MR, Piacente S, Masullo M. Development of a New Extraction Method for Pomegranate and Metabolite Profiling by a LC-MS and 1H NMR Combined Approach. Foods. 2024; 13(10):1429. https://doi.org/10.3390/foods13101429
Chicago/Turabian StylePolcaro, Luciana Maria, Marzieh Rahmani Samani, Sonia Piacente, and Milena Masullo. 2024. "Development of a New Extraction Method for Pomegranate and Metabolite Profiling by a LC-MS and 1H NMR Combined Approach" Foods 13, no. 10: 1429. https://doi.org/10.3390/foods13101429