Enhancing Escherichia coli Inactivation: Synergistic Mechanism of Ultraviolet Light and High-Voltage Electric Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agar Plate
2.2. Bacterial Strains
2.3. Disinfection Treatments
2.3.1. Ultraviolet Treatment
2.3.2. High-Voltage Electric Field Treatments
2.3.3. Combined Treatment
2.4. Characterization
2.4.1. Bactericidal Efficacy of UV, HVEF, and Combined Treatment on E. coli
2.4.2. The Integrity of Cell Membrane
Scanning Electron Microscopy (SEM)
Transmission Electron Microscopy (TEM)
Bicinchoninic Acid Assay
2.4.3. The Permeability of Cell Membrane
Propidium Iodide Staining Assays
2.4.4. The Oxidative Damage of Cell Membrane
Reactive Oxygen Assay
2.4.5. The Apoptosis of E. coli
2.4.6. The Fluidity of the Cell Membrane
2.5. Statistical Analysis
3. Results and Discussion
3.1. Inactivation of E. coli under Single or Combined Processes of UV and HVEF
3.2. The Integrity of the Cell Membrane
3.2.1. SEM
3.2.2. TEM
3.2.3. Protein Leakage
3.3. The Permeability of Cell Membrane
Propidium Iodinated Fluorescence
3.4. The Oxidative Damage of Cell Membrane
3.5. The Apoptosis of E. coli
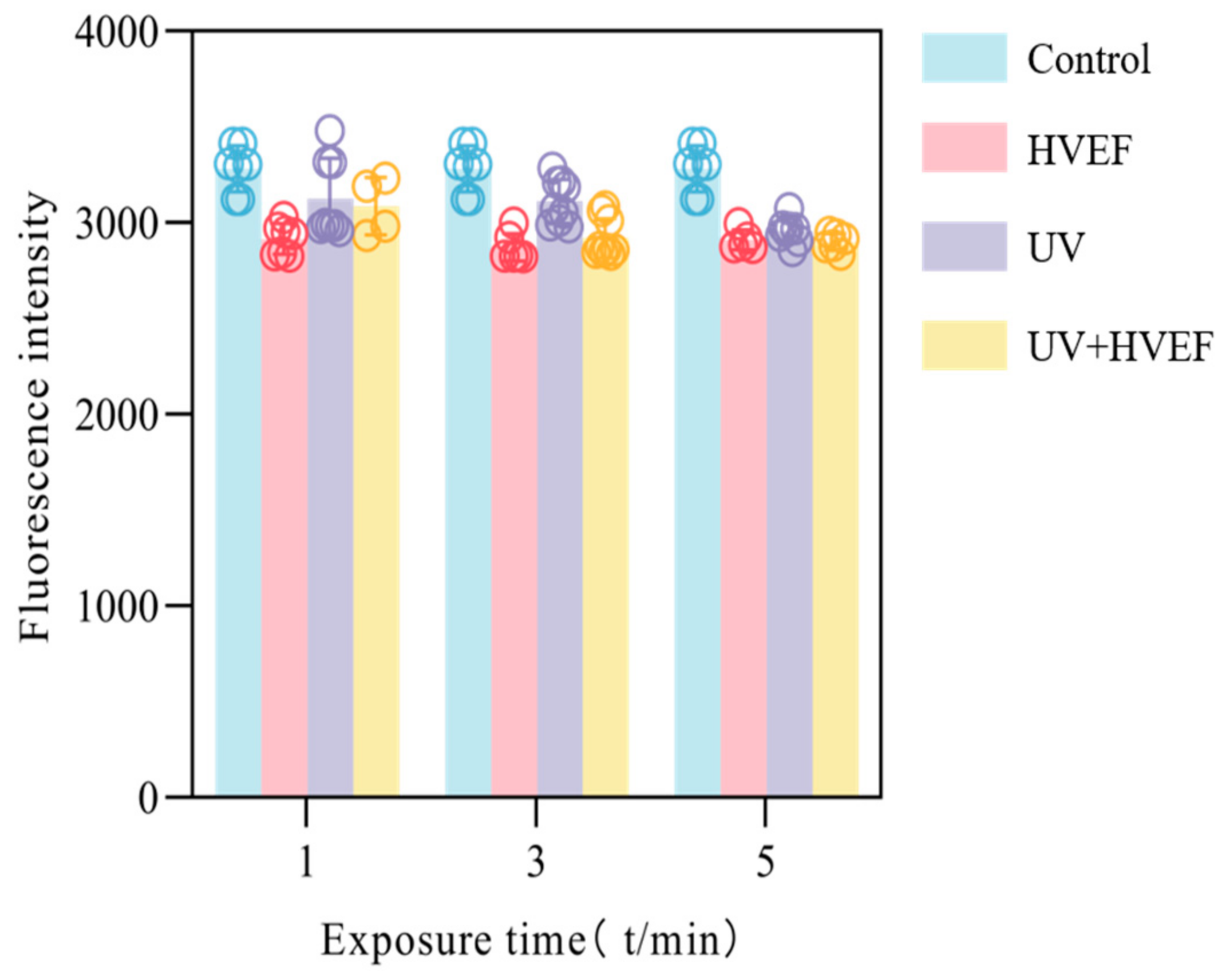
3.6. The Fluidity of Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sholtes, K.A.; Lowe, K.; Walters, G.W.; Sobsey, M.D.; Linden, K.G.; Casanova, L.M. Comparison of Ultraviolet Light-Emitting Diodes and Low-Pressure Mercury-Arc Lamps for Disinfection of Water. Environ. Technol. 2016, 37, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photon. 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-642-01998-2. [Google Scholar]
- Zou, X.-Y.; Lin, Y.-L.; Xu, B.; Cao, T.-C.; Tang, Y.-L.; Pan, Y.; Gao, Z.-C.; Gao, N.-Y. Enhanced Inactivation of E. coli by Pulsed UV-LED Irradiation during Water Disinfection. Sci. Total Environ. 2019, 650, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Beber de Souza, J.; Queiroz Valdez, F.; Jeranoski, R.F.; Vidal, C.M.d.S.; Cavallini, G.S. Water and Wastewater Disinfection with Peracetic Acid and UV Radiation and Using Advanced Oxidative Process PAA/UV. Int. J. Photoenergy 2015, 2015, e860845. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhang, X.; Wang, X.; Lv, L. Combined Applications of UV and Chlorine on Antibiotic Resistance Control: A Critical Review. Environ. Res. 2024, 243, 117884. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhao, R.; Liu, Q.; Yan, H.; Zhang, Y.; Wang, S.; Yuan, Y. Antibacterial Activity and Mechanism of High Voltage Electrostatic Field (HVEF) against Staphylococcus Aureus in Medium Plates and Food Systems. Food Control 2021, 120, 107566. [Google Scholar] [CrossRef]
- Wei, S.; Chen, T.; Hou, H.; Xu, Y. Recent Advances in Electrochemical Sterilization. J. Electroanal. Chem. 2023, 937, 117419. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of Cell Membranes. In Electroporation and Electrofusion in Cell Biology; Neumann, E., Sowers, A.E., Jordan, C.A., Eds.; Springer: Boston, MA, USA, 1989; pp. 149–163. ISBN 978-1-4899-2528-2. [Google Scholar]
- Singh, A.; Orsat, V.; Raghavan, V. A Comprehensive Review on Electrohydrodynamic Drying and High-Voltage Electric Field in the Context of Food and Bioprocessing. Dry. Technol. 2012, 30, 1812–1820. [Google Scholar] [CrossRef]
- Huang, H.; Sun, W.; Xiong, G.; Shi, L.; Jiao, C.; Wu, W.; Li, X.; Qiao, Y.; Liao, L.; Ding, A.; et al. Effects of HVEF Treatment on Microbial Communities and Physicochemical Properties of Catfish Fillets during Chilled Storage. LWT 2020, 131, 109667. [Google Scholar] [CrossRef]
- Cramariuc, R.; Popa, M.; Tudorache, A.; Brînduşe, E.; Kontek, A.; Mitelut, A.; Fotescu, L.; Cramariuc, B.; Geicu, M.; Nisiparu, L. PEF and UV Combined System for Pathogen Microorganisms Inactivation in Liquid Food Products. J. Phys. Conf. Ser. 2011, 301, 012010. [Google Scholar] [CrossRef]
- Gachovska, T.K.; Kumar, S.; Thippareddi, H.; Subbiah, J.; Williams, F. Ultraviolet and Pulsed Electric Field Treatments Have Additive Effect on Inactivation of E. coli in Apple Juice. J. Food Sci. 2008, 73, M412–M417. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, I.M.; Noci, F.; Muñoz, A.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. Impact of Selected Combinations of Non-Thermal Processing Technologies on the Quality of an Apple and Cranberry Juice Blend. Food Chem. 2011, 124, 1387–1392. [Google Scholar] [CrossRef]
- Wu, S.; Xu, X.; Yang, N.; Jin, Y.; Jin, Z.; Xie, Z. Inactivation of Escherichia coli O157:H7 in Apple Juice via Induced Electric Field (IEF) and Its Bactericidal Mechanism. Food Microbiol. 2022, 102, 103928. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Ngo, H.H.; Mao, Y.; Cao, K.; Shi, Q.; Lu, Y.; Hu, H.-Y. Comparison of Inactivation Characteristics between Gram-Positive and Gram-Negative Bacteria in Water by Synergistic UV and Chlorine Disinfection. Environ. Pollut. 2023, 333, 122007. [Google Scholar] [CrossRef] [PubMed]
- Batool, A. Augmented Antibacterial Activity of Cefazolin with Silver Nanoparticles against Staphylococcus Aureus and Escherichia coli. J. Drug Deliv. Sci. Technol. 2023, 85, 104550. [Google Scholar] [CrossRef]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV Light Emitting Diodes to Batch and Flow-through Water Disinfection Systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Martín, O.; Qin, B.L.; Chang, F.J.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Escherichia coli in Skim Milk by High Intensity Pulsed Electric Fields. J. Food Process Eng. 1997, 20, 317–336. [Google Scholar] [CrossRef]
- Cai, X.; Wang, X.; Chen, Y.; Wang, Y.; Song, D.; Gu, Q. A Natural Biopreservative: Antibacterial Action and Mechanisms of Chinese Litsea Mollis Hemsl. Extract against Escherichia coli DH5α and Salmonella spp. J. Dairy Sci. 2019, 102, 9663–9673. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Q.; Zhang, T.; Chen, X.; Wu, Z.; Zhang, N.; Shao, Y.; Cheng, Y. Antibacterial Activity and Mechanism of Green Tea Polysaccharide Conjugates against Escherichia coli. Ind. Crops Prod. 2020, 152, 112464. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Li, C.; Hua, S.; Sun, C.; Huang, L. Antibacterial Mechanism of Vanillin against Escherichia coli O157: H7. Heliyon 2023, 9, e19280. [Google Scholar] [CrossRef] [PubMed]
- Delsart, C.; Grimi, N.; Boussetta, N.; Miot Sertier, C.; Ghidossi, R.; Vorobiev, E.; Mietton Peuchot, M. Impact of Pulsed-Electric Field and High-Voltage Electrical Discharges on Red Wine Microbial Stabilization and Quality Characteristics. J. Appl. Microbiol. 2016, 120, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Paul, B.K.; Guchhait, N. Photophysics of DNA Staining Dye Propidium Iodide Encapsulated in Bio-Mimetic Micelle and Genomic Fish Sperm DNA. J. Photochem. Photobiol. B Biol. 2012, 109, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Feng, X.; Hou, Y.; Sun, A.; Wang, R. Cold Plasma Jet with Dielectric Barrier Configuration: Investigating Its Effect on the Cell Membrane of E. coli and S. cerevisiae and Its Impact on the Quality of Chokeberry Juice. LWT 2021, 136, 110223. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring Reactive Species and Oxidative Damage in Vivo and in Cell Culture: How Should You Do It and What Do the Results Mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive Oxygen Species in Plant Development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cheng, J.-H.; Lv, X.; Sun, D.-W. Assessing the Inactivation Efficiency of Ar/O2 Plasma Treatment against Listeria Monocytogenes Cells: Sublethal Injury and Inactivation Kinetics. LWT 2019, 111, 318–327. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E.; Denlinger, D.L. High Resistance to Oxidative Damage in the Antarctic Midge Belgica Antarctica, and Developmentally Linked Expression of Genes Encoding Superoxide Dismutase, Catalase and Heat Shock Proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- Wang, G.; Huang, J.; Gao, W.; Lu, J.; Li, J.; Liao, R.; Jaleel, C.A. The Effect of High-Voltage Electrostatic Field (HVEF) on Aged Rice (Oryza sativa L.) Seeds Vigor and Lipid Peroxidation of Seedlings. J. Electrostat. 2009, 67, 759–764. [Google Scholar] [CrossRef]
- Kawano, A.; Yamasaki, R.; Sakakura, T.; Takatsuji, Y.; Haruyama, T.; Yoshioka, Y.; Ariyoshi, W. Reactive Oxygen Species Penetrate Persister Cell Membranes of Escherichia coli for Effective Cell Killing. Front. Cell. Infect. Microbiol. 2020, 10, 496. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Ahn, J.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Ding, T. Preceding Treatment of Non-Thermal Plasma (NTP) Assisted the Bactericidal Effect of Ultrasound on Staphylococcus aureus. Food Control 2018, 90, 241–248. [Google Scholar] [CrossRef]
- Villegas, J.; Schulz, M.; Soto, L.; Sanchez, R. Bacteria Induce Expression of Apoptosis in Human Spermatozoa. Apoptosis 2005, 10, 105–110. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Shen, X.; Zhang, S.; Chen, X. Lethal and Sublethal Injury and Kinetics of Escherichia coli, Listeria Monocytogenes and Staphylococcus Aureus in Milk by Pulsed Electric Fields. Food Control 2013, 32, 6–12. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liang, Y.; Pan, D.; Bai, S.; Wen, D.; Tang, M.; Song, H.; Guo, X.; Han, H. Enhancing Escherichia coli Inactivation: Synergistic Mechanism of Ultraviolet Light and High-Voltage Electric Field. Foods 2024, 13, 1343. https://doi.org/10.3390/foods13091343
Zhang Y, Liang Y, Pan D, Bai S, Wen D, Tang M, Song H, Guo X, Han H. Enhancing Escherichia coli Inactivation: Synergistic Mechanism of Ultraviolet Light and High-Voltage Electric Field. Foods. 2024; 13(9):1343. https://doi.org/10.3390/foods13091343
Chicago/Turabian StyleZhang, Yihan, Yun Liang, Di Pan, Shupei Bai, Diya Wen, Min Tang, Hua Song, Xuan Guo, and Hao Han. 2024. "Enhancing Escherichia coli Inactivation: Synergistic Mechanism of Ultraviolet Light and High-Voltage Electric Field" Foods 13, no. 9: 1343. https://doi.org/10.3390/foods13091343





