A Cold Flow Model of Interconnected Slurry Bubble Columns for Sorption-Enhanced Fischer–Tropsch Synthesis
Abstract
:1. Introduction
2. Materials, Methods and Methodology
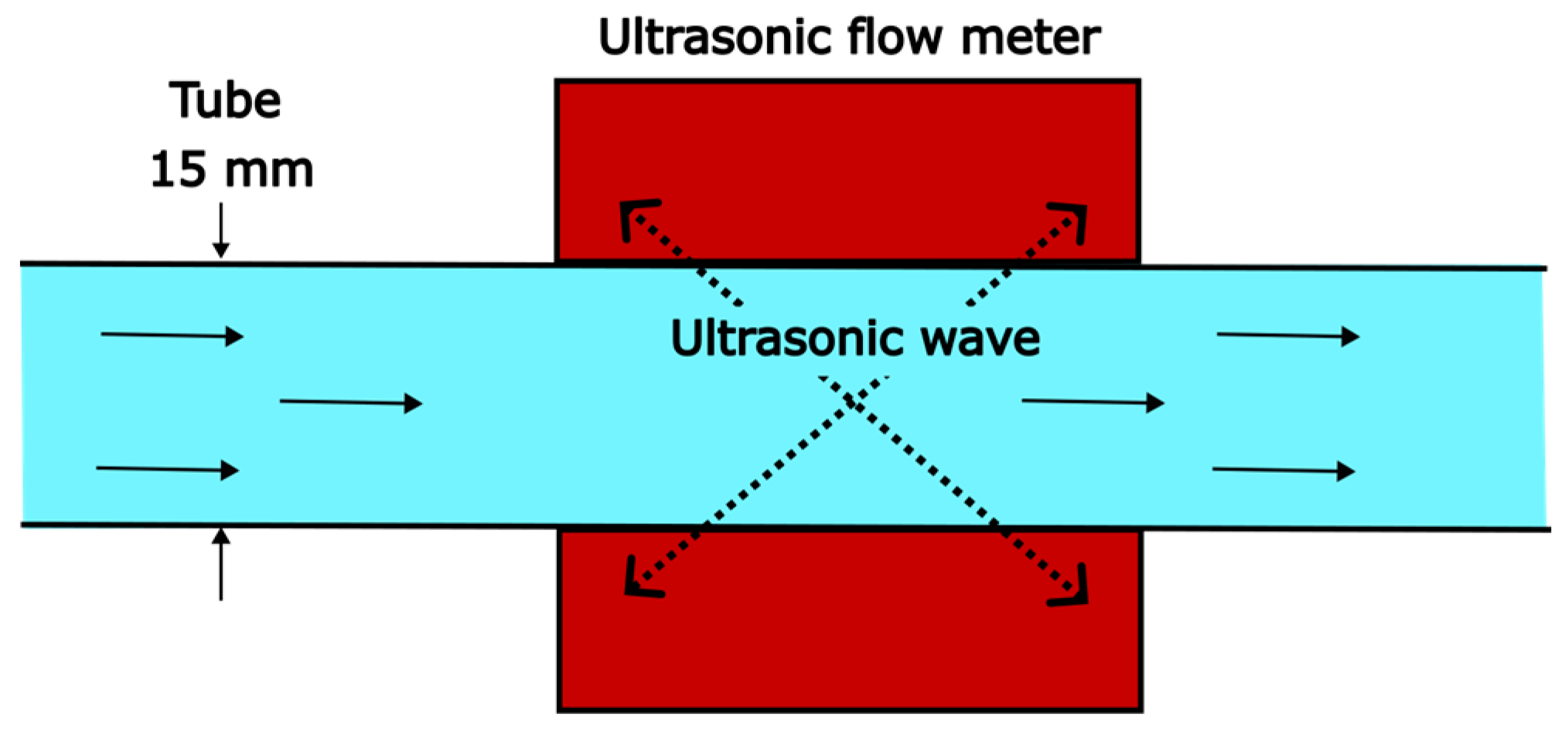
2.1. Experimental Setup and Measuring Methods
2.2. AI Modelling
- Preprocessing: This step involves extracting, cleaning and separating the experimental data, which is comprised of 95 data points. The data are divided into features and labels. In this work, the features include gas volume flow in both BC, liquid exit height, tube inner diameter and electrical conductivity. Since only LCR is supposed to be predicted, the number of labels is one.
- All features are normalized with a MinMaxScaler from sklearn within the interval [0, 1], using Equation (7). This procedure is essential to avoid any potential influence of the differing value ranges of the features. Each normalized value of a feature ) is calculated using its maximum () and minimum () values alongside the actual value ().
- 3.
- This equation is suitable as it retains the relative scaling between all feature values. The last step of preprocessing involves splitting the data into training and testing data. Typically, 70–80% of the original data are used for training, while the remaining 20–30% are reserved for evaluating model’s accuracy [27].
- 4.
- Training: In this step, the models are trained. A multilayer perceptron (MLP) and EXT model are developed in the present work. Both are explained in greater detail in Section 2.2.1 and Section 2.2.2. Identical training data are supplied to both models and optimal hyperparameters (HPs) are determined using the GridSearchCV method from sklearn. This method has the option of performing a k-fold cross-validation to enhance the model’s accuracy. In this work, a 5-fold cross-validation is performed for each fit in the gird search algorithm. Defined HPs and their ranges for both models are listed in Table 1 and Table 2. Both models are optimized with the mean squared error (MSE) as presented in Equation (8).
- 5.
- MSE is defined as the sum of squared differences between the experimental value () and the predicted value () for n predictions. The fits with the lowest MSE are used in the following step. Parity plots of the training data are plotted with matplotlib.
- 6.
- Testing and Evaluation: In this step, the remaining unknown testing data are used to assess the model’s accuracy. Parity plots are generated using matplotlib. For comparison, MAPE and R2 are calculated and saved. MAPE is defined as the sum of differences between the experimental value () and the predicted value () divided by the experimental value for n predictions (Equation (9)).
- 7.
- R2 is defined as the sum of residual squares divided by the total sum of squares (Equation (10)). The sum of residual squares is calculated by the sum of squared differences between the experimental () and predicted value . The sum of total squares contains the sum of squared differences between the experimental value () and the mean experimental value .
- The closer the value of is to one, the better the predicted data represent the experimental values. All models, along with the best HPs, are saved using joblib.
2.2.1. Multilayer Perceptron
| Hyperparameter | Range | Final Hyperparameter |
|---|---|---|
| Batch size | 5, 10, 20 | 10 |
| Number of hidden layers | 1, 2, 3 | 3 |
| Number of neurons | 8, 16, 64, 128, 512 | 128 |
| Number of epochs | 100, 300, 500, 1000, 1500 | 1500 |
| Learning rate | 10−4, 10−3, 10−2 | 10−3 |
| Activation function | Rectified linear unit, hyperbolic tangent, sigmoid | Rectified linear unit |
2.2.2. Extra Trees
| Hyperparameter | Range | Final Hyperparameter |
|---|---|---|
| n_estimators | 10, 50, 100, 200, 500 | 100 |
| max_depth | None, 5, 10, 20, 30 | 5 |
| max_features | None, 2, sqrt, log2, 0.3 | None |
| min_sample_split | 5, 10, 20 | 5 |
3. Results and Discussion
3.1. Cold Model Studies
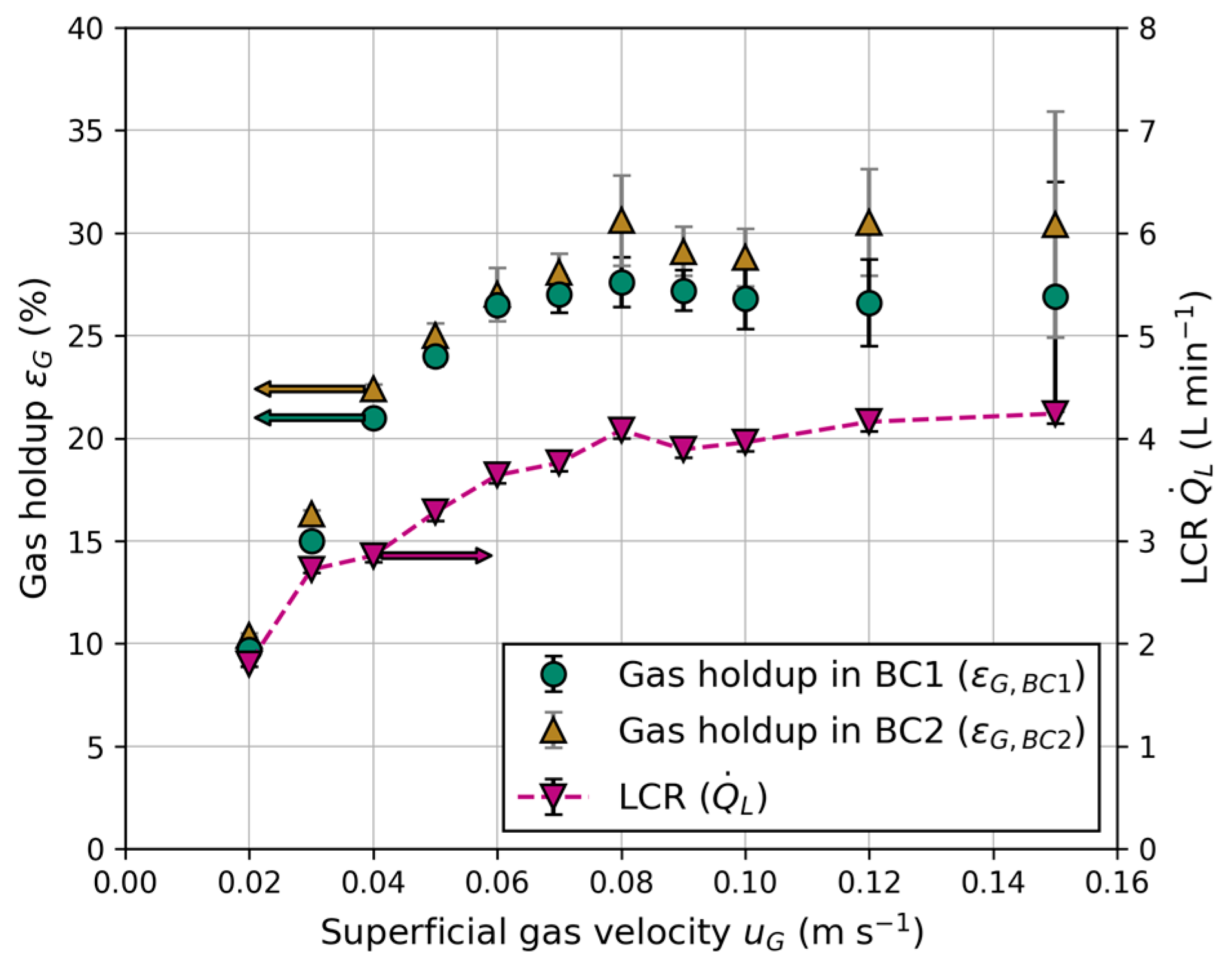
3.1.1. Effect of Superficial Gas Velocity on LCR
3.1.2. Effect of Water Quality and Liquid Height on LCR
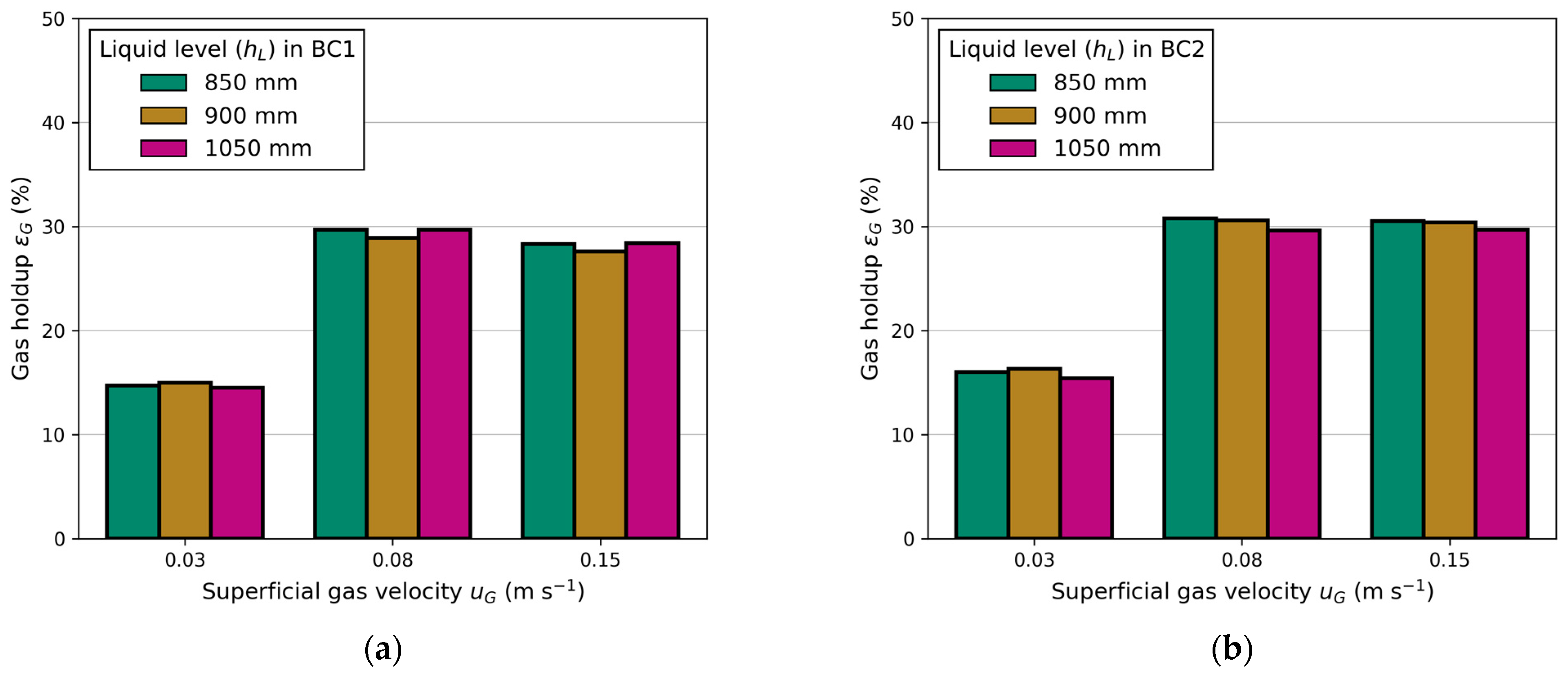
3.1.3. Effect of Liquid Exit Height and Tube Diameter on LCR
3.1.4. Identifying the Main Influencing Parameter on LCR for SE FT Synthesis
3.2. AI Modelling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Reference Point (m s−1) | Run Time (h) | LCR (L min−1) | Standard Deviation of Reference Points (L min−1) |
|---|---|---|---|
| 0.03 | 20 | 2.68 ± 0.04 | 0.04 |
| 3 | 2.7 ± 0.05 | ||
| 8 | 2.7 ± 0.05 | ||
| 0.06 | 14 | 3.64 ± 0.14 | 0.04 |
| 2 | 3.6 ± 0.15 | ||
| 6 | 3.68 ± 0.15 |
Appendix B
Appendix C
| LCR | |||||||
|---|---|---|---|---|---|---|---|
| Correlation coefficient for LCR (-) | 0.176 | 0.91 | 0.002 | 0.178 | 0.485 | 0.908 | 1 |
Appendix D
| Features | Label | |||||
|---|---|---|---|---|---|---|
| Index * | Gas Volume Flow BC1 (m3 h−1) | Gas Volume Flow BC2 (m3 h−1) | Liquid Exit Height (mm) | Tube Inner Diameter (mm) | Electrical Conductivity (μS cm−1) | LCR (mL min−1) |
| 1 | 1.70 | 3.33 | 800 | 15 | 250 | 3608.533 |
| 2 | 0.85 | 1.66 | 800 | 15 | 250 | 2686.062 |
| 3 | 1.70 | 3.33 | 800 | 15 | 250 | 3639.435 |
| 4 | 0.85 | 1.66 | 800 | 15 | 250 | 2716.402 |
| 5 | 1.70 | 3.33 | 800 | 15 | 250 | 3652.783 |
| 6 | 0.85 | 1.66 | 800 | 15 | 250 | 2720.485 |
| 7 | 1.70 | 3.33 | 800 | 15 | 250 | 3642.520 |
| 8 | 0.85 | 1.66 | 800 | 15 | 250 | 2688.969 |
| 9 | 1.70 | 3.33 | 800 | 15 | 250 | 3763.355 |
| 10 | 0.85 | 1.66 | 800 | 15 | 250 | 2733.940 |
| 11 | 1.70 | 3.33 | 800 | 15 | 250 | 3786.024 |
| 12 | 2.26 | 4.43 | 800 | 15 | 250 | 4045.343 |
| 13 | 2.26 | 4.43 | 800 | 15 | 250 | 4156.077 |
| 14 | 0.85 | 1.66 | 800 | 15 | 250 | 2680.508 |
| 15 | 2.26 | 4.43 | 800 | 15 | 250 | 4092.790 |
| 16 | 1.13 | 2.22 | 800 | 15 | 250 | 2854.361 |
| 17 | 1.70 | 3.33 | 800 | 15 | 250 | 3640.642 |
| 18 | 0.85 | 1.66 | 800 | 15 | 250 | 2695.432 |
| 19 | 1.41 | 2.77 | 800 | 15 | 250 | 3243.155 |
| 20 | 1.98 | 3.88 | 800 | 15 | 250 | 3744.599 |
| 21 | 2.54 | 4.99 | 800 | 15 | 250 | 3893.254 |
| 22 | 0.57 | 1.11 | 800 | 15 | 250 | 1874.593 |
| 23 | 2.83 | 5.54 | 800 | 15 | 250 | 3960.174 |
| 24 | 3.39 | 6.65 | 800 | 15 | 250 | 4166.623 |
| 25 | 4.24 | 8.31 | 800 | 15 | 250 | 4266.508 |
| 26 | 0.42 | 0.83 | 800 | 15 | 250 | 946.540 |
| 27 | 2.26 | 4.43 | 800 | 15 | 250 | 3705.236 |
| 28 | 0.35 | 0.69 | 800 | 15 | 1 | 1137.410 |
| 29 | 2.26 | 4.43 | 800 | 15 | 1 | 3993.033 |
| 30 | 1.70 | 3.33 | 800 | 15 | 1 | 3280.818 |
| 31 | 0.85 | 1.66 | 800 | 15 | 1 | 2588.364 |
| 32 | 1.41 | 2.77 | 800 | 15 | 1 | 3107.839 |
| 33 | 1.98 | 3.88 | 800 | 15 | 1 | 3444.135 |
| 34 | 2.83 | 5.54 | 800 | 15 | 1 | 3759.801 |
| 35 | 2.26 | 4.43 | 800 | 15 | 1 | 3550.832 |
| 36 | 3.39 | 6.65 | 800 | 15 | 1 | 3883.723 |
| 37 | 4.24 | 8.31 | 800 | 15 | 1 | 3950.199 |
| 38 | 2.54 | 4.99 | 800 | 15 | 1 | 3619.556 |
| 39 | 1.70 | 3.33 | 800 | 15 | 1 | 3078.155 |
| 40 | 0.85 | 1.66 | 800 | 15 | 1 | 2526.910 |
| 41 | 1.13 | 2.22 | 800 | 15 | 1 | 2805.389 |
| 42 | 1.70 | 3.33 | 800 | 15 | 1 | 2992.969 |
| 43 | 0.57 | 1.11 | 800 | 15 | 1 | 1810.442 |
| 44 | 1.70 | 3.33 | 800 | 13 | 250 | 3511.756 |
| 45 | 0.85 | 1.66 | 800 | 13 | 250 | 2567.310 |
| 46 | 2.26 | 4.43 | 800 | 13 | 250 | 3597.505 |
| 47 | 2.83 | 5.54 | 800 | 13 | 250 | 3739.891 |
| 48 | 3.39 | 6.65 | 800 | 13 | 250 | 3882.929 |
| 49 | 4.24 | 8.31 | 800 | 13 | 250 | 3980.338 |
| 50 | 0.57 | 1.11 | 800 | 13 | 250 | 1729.601 |
| 51 | 2.54 | 4.99 | 800 | 13 | 250 | 3551.215 |
| 52 | 1.13 | 2.22 | 800 | 13 | 250 | 2944.572 |
| 53 | 1.70 | 3.33 | 800 | 13 | 250 | 3647.495 |
| 54 | 0.85 | 1.66 | 800 | 13 | 250 | 2576.687 |
| 55 | 1.41 | 2.77 | 800 | 13 | 250 | 2882.141 |
| 56 | 0.85 | 1.66 | 800 | 15 | 250 | 2557.055 |
| 57 | 2.26 | 4.43 | 800 | 15 | 250 | 3989.512 |
| 58 | 4.24 | 8.31 | 800 | 15 | 250 | 4256.377 |
| 59 | 0.85 | 1.66 | 800 | 15 | 250 | 2609.819 |
| 60 | 2.26 | 4.43 | 800 | 15 | 250 | 3993.156 |
| 61 | 4.24 | 8.31 | 800 | 15 | 250 | 4080.244 |
| 62 | 0.85 | 1.66 | 400 | 15 | 250 | 1075.999 |
| 63 | 1.70 | 3.33 | 400 | 15 | 250 | 1330.241 |
| 64 | 2.26 | 4.43 | 400 | 15 | 250 | 1231.533 |
| 65 | 3.39 | 6.65 | 400 | 15 | 250 | 1443.683 |
| 66 | 4.24 | 8.31 | 400 | 15 | 250 | 1401.415 |
| 67 | 0.85 | 1.66 | 600 | 15 | 250 | 1526.860 |
| 68 | 1.70 | 3.33 | 600 | 15 | 250 | 1943.773 |
| 69 | 2.26 | 4.43 | 600 | 15 | 250 | 1950.439 |
| 70 | 3.39 | 6.65 | 600 | 15 | 250 | 2541.098 |
| 71 | 4.24 | 8.31 | 600 | 15 | 250 | 2699.396 |
| 72 | 0.85 | 1.66 | 800 | 15 | 250 | 2567.234 |
| 73 | 2.26 | 4.43 | 800 | 15 | 250 | 3598.699 |
| 74 | 2.83 | 5.54 | 800 | 15 | 250 | 3738.784 |
| 75 | 3.39 | 6.65 | 800 | 15 | 250 | 3880.856 |
| 76 | 4.24 | 8.31 | 800 | 15 | 250 | 3975.001 |
| 77 | 2.54 | 4.99 | 800 | 15 | 250 | 3549.043 |
| 78 | 1.13 | 2.22 | 800 | 15 | 250 | 2853.993 |
| 79 | 1.70 | 3.33 | 800 | 15 | 250 | 3568.206 |
| 80 | 0.85 | 1.66 | 800 | 15 | 250 | 2543.355 |
| 81 | 1.41 | 2.77 | 800 | 15 | 250 | 2881.274 |
| 82 | 1.98 | 3.88 | 800 | 15 | 250 | 3143.327 |
| 83 | 1.70 | 3.33 | 800 | 15 | 250 | 3764.697 |
| 84 | 0.85 | 1.66 | 800 | 15 | 250 | 2571.983 |
| 85 | 2.26 | 4.43 | 800 | 15 | 250 | 3993.033 |
| 86 | 5.65 | 11.08 | 800 | 15 | 250 | 4023.701 |
| 87 | 1.70 | 4.99 | 800 | 15 | 250 | 4021.535 |
| 88 | 0.85 | 1.66 | 800 | 15 | 250 | 2691.569 |
| 89 | 0.85 | 3.33 | 800 | 15 | 250 | 3099.100 |
| 90 | 0.85 | 4.99 | 800 | 15 | 250 | 3209.846 |
| 91 | 1.70 | 1.66 | 800 | 15 | 250 | 3397.428 |
| 92 | 2.54 | 1.66 | 800 | 15 | 250 | 3476.905 |
| 93 | 2.54 | 3.33 | 800 | 15 | 250 | 4122.558 |
| 94 | 1.70 | 3.33 | 800 | 15 | 250 | 3645.568 |
| 95 | 0.85 | 1.66 | 800 | 15 | 250 | 2682.548 |
Appendix E

References
- Van Kampen, J.; Boon, J.; Van Berkel, F.; Vente, J.; Van Sint Annaland, M. Steam separation enhanced reactions: Review and outlook. Chem. Eng. J. 2019, 374, 1286–1303. [Google Scholar] [CrossRef]
- Kiefer, F.; Nikolic, M.; Borgschulte, A.; Dimopoulos Eggenschwiler, P. Sorption-enhanced methane synthesis in fixed-bed reactors. Chem. Eng. J. 2022, 449, 137872. [Google Scholar] [CrossRef]
- Van Kampen, J.; Boon, J.; Vente, J.; Van Sint Annaland, M. Sorption enhanced dimethyl ether synthesis for high efficiency carbon conversion: Modelling and cycle design. J. CO2 Util. 2020, 37, 295–308. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Patterson, P.M.; Li, J.; Sanchez, L.; Davis, B.H. Fischer–Tropsch synthesis XAFS. Appl. Catal. Gen. 2003, 247, 335–343. [Google Scholar] [CrossRef]
- Li, J.; Jacobs, G.; Das, T.; Zhang, Y.; Davis, B. Fischer–Tropsch synthesis: Effect of water on the catalytic properties of a Co/SiO2 catalyst. Appl. Catal. Gen. 2002, 236, 67–76. [Google Scholar] [CrossRef]
- Storsater, S.; Borg, O.; Blekkan, E.; Holmen, A. Study of the effect of water on Fischer–Tropsch synthesis over supported cobalt catalysts. J. Catal. 2005, 231, 405–419. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Tu, M.; Ojeda, M.P.; Pinna, D.; Iglesia, E. An Investigation of the Effects of Water on Rate and Selectivity for the Fischer–Tropsch Synthesis on Cobalt-Based Catalysts. J. Catal. 2002, 211, 422–433. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes, 1st ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-0-471-45713-8. [Google Scholar]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer–Tropsch synthesis with in situ H2O removal—Directions of membrane development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Gavrilović, L.; Kazi, S.S.; Oliveira, A.; Encinas, O.L.I.; Blekkan, E.A. Sorption-enhanced Fischer-Tropsch synthesis—Effect of water removal. Catal. Today 2024, 432, 114614. [Google Scholar] [CrossRef]
- Delmelle, R.; Duarte, R.B.; Franken, T.; Burnat, D.; Holzer, L.; Borgschulte, A.; Heel, A. Development of improved nickel catalysts for sorption enhanced CO2 methanation. Int. J. Hydrogen Energy 2016, 41, 20185–20191. [Google Scholar] [CrossRef]
- Borgschulte, A.; Callini, E.; Stadie, N.; Arroyo, Y.; Rossell, M.D.; Erni, R.; Geerlings, H.; Züttel, A.; Ferri, D. Manipulating the reaction path of the CO2 hydrogenation reaction in molecular sieves. Catal. Sci. Technol. 2015, 5, 4613–4621. [Google Scholar] [CrossRef]
- Ghodhbene, M.; Bougie, F.; Fongarland, P.; Iliuta, M.C. Hydrophilic zeolite sorbents for In-situ water removal in high temperature processes. Can. J. Chem. Eng. 2017, 95, 1842–1849. [Google Scholar] [CrossRef]
- Krishna, R. A Scale-Up Strategy for a Commercial Scale Bubble Column Slurry Reactor for Fischer-Tropsch Synthesis. Oil Gas Sci. Technol. 2000, 55, 359–393. [Google Scholar] [CrossRef]
- Krishna, R.; Sie, S.T. Design and scale-up of the Fischer–Tropsch bubble column slurry reactor. Fuel Process. Technol. 2000, 64, 73–105. [Google Scholar] [CrossRef]
- Krishna, R.; Van Baten, J.M. A Strategy for Scaling Up the Fischer–Tropsch Bubble Column Slurry Reactor. Top. Catal. 2003, 26, 21–28. [Google Scholar] [CrossRef]
- De Deugd, R.M.; Kapteijn, F.; Moulijn, J.A. Trends in Fischer–Tropsch Reactor Technology—Opportunities for Structured Reactors. Top. Catal. 2003, 26, 29–39. [Google Scholar] [CrossRef]
- Chisti, M.Y.; Moo-Young, M. Airlift reactors: Characteristics, applications and design considerations. Chem. Eng. Commun. 1987, 60, 195–242. [Google Scholar] [CrossRef]
- Jafarian, M.; Chisti, Y.; Nathan, G.J. Gas-lift circulation of a liquid between two inter-connected bubble columns. Chem. Eng. Sci. 2020, 218, 115574. [Google Scholar] [CrossRef]
- Hsu, C.; Dudukovie, M.P. Gas holdup and liquid recirculation in gas-lift reactors. Chem. Eng. Sci. 1980, 35, 135–141. [Google Scholar] [CrossRef]
- Camarasa, E.; Carvalho, E.; Meleiro, L.A.C.; Maciel Filho, R.; Domingues, A.; Wild, G.; Poncin, S.; Midoux, N.; Bouillard, J. A hydrodynamic model for air-lift reactors. Chem. Eng. Process. Process Intensif. 2001, 40, 121–128. [Google Scholar] [CrossRef]
- Verlaan, P.; Tramper, J.; Van’t Reit, K.; Luyben, K.C.H.A.M. A hydrodynamic model for an airlift-loop bioreactor with external loop. Chem. Eng. J. 1986, 33, B43–B53. [Google Scholar] [CrossRef]
- Glennon, B.; Al-Masry, W.; MacLoughlin, P.F.; Malone, D.M. Hydrodynamic modellin in an air-lift loop reactor. Chem. Eng. Commun. 1993, 121, 181–192. [Google Scholar] [CrossRef]
- Zuber, N.; Findlay, J.A. Average Volumetric Concentration in Two-Phase Flow Systems. J. Heat Transf. 1965, 87, 453–468. [Google Scholar] [CrossRef]
- Chisti, M.Y.; Halard, B.; Moo-Young, M. Liquid circulation in airlift reactors. Chem. Eng. Sci. 1988, 43, 451–457. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Cheng, Y.-L. Gas holdup and liquid velocity in three-phase internal-loop airlift reactors. Chem. Eng. Sci. 1997, 52, 3949–3960. [Google Scholar] [CrossRef]
- Amiri, S.; Mehrnia, M.R.; Barzegari, D.; Yazdani, A. An artificial neural network for prediction of gas holdup in bubble columns with oily solutions. Neural Comput. Appl. 2011, 20, 487–494. [Google Scholar] [CrossRef]
- Baawain, M.S.; Gamal El-Din, M.; Smith, D.W. Artificial Neural Networks Modeling of Ozone Bubble Columns: Mass Transfer Coefficient, Gas Hold-Up, and Bubble Size. Ozone Sci. Eng. 2007, 29, 343–352. [Google Scholar] [CrossRef]
- Behkish, A.; Lemoine, R.; Sehabiague, L.; Oukaci, R.; Morsi, B.I. Prediction of the Gas Holdup in Industrial-Scale Bubble Columns and Slurry Bubble Column Reactors Using Back-Propagation Neural Networks. Int. J. Chem. React. Eng. 2005, 3, 1. [Google Scholar] [CrossRef]
- Shaikh, A.; Al-Dahhan, M. Development of an artificial neural network correlation for prediction of overall gas holdup in bubble column reactors. Chem. Eng. Process. Process Intensif. 2003, 42, 599–610. [Google Scholar] [CrossRef]
- Hazare, S.R.; Patil, C.S.; Vala, S.V.; Joshi, A.J.; Joshi, J.B.; Vitankar, V.S.; Patwardhan, A.W. Predictive analysis of gas hold-up in bubble column using machine learning methods. Chem. Eng. Res. Des. 2022, 184, 724–739. [Google Scholar] [CrossRef]
- Erickson, L.E. Airlift bioreactors, by M.Y. Chisti. First edition, 1989, 345 pages. Elsevier applied science, London, England and New York, USA $74.00 (U.S.). Can. J. Chem. Eng. 1990, 68, 349. [Google Scholar] [CrossRef]
- Pirdashti, M.; Curteanu, S.; Kamangar, M.H.; Hassim, M.H.; Khatami, M.A. Artificial neural networks: Applications in chemical engineering. Rev. Chem. Eng. 2013, 29, 205–239. [Google Scholar] [CrossRef]
- Verma, A.K.; Mohamad, E.T.; Bhatawdekar, R.M.; Raina, A.K.; Khandelwal, M.; Armaghani, D.; Sarkar, K. (Eds.) Proceedings of Geotechnical Challenges in Mining, Tunneling and Underground Infrastructures: ICGMTU, 20 December 2021; Lecture Notes in Civil Engineering; Springer Nature Singapore: Singapore, 2022; Volume 228, ISBN 9789811697692. [Google Scholar]
- Urseanu, M.I. Scaling up Bubble Column Reactors. Ph.D. Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 2000. Available online: https://dare.uva.nl/search?identifier=a4a97721-8be2-4f3b-becf-f081292595fc (accessed on 22 February 2024).
- Hikita, H.; Asai, S.; Tanigawa, K.; Segawa, K.; Kitao, M. Gas Hold-up in Bubble Columns. Chem. Eng. J. 1980, 20, 59–67. [Google Scholar] [CrossRef]
- Deen, N.G.; Mudde, R.F.; Kuipers, J.A.M.; Zehner, P.; Kraume, M. Bubble Columns. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-3-527-30385-4. [Google Scholar]
- Gemello, L.; Plais, C.; Augier, F.; Cloupet, A.; Marchisio, D.L. Hydrodynamics and bubble size in bubble columns: Effects of contaminants and spargers. Chem. Eng. Sci. 2018, 184, 93–102. [Google Scholar] [CrossRef]
- Chisti, M.Y.; Moo-Young, M. Gas holdup in pneumatic reactors. Chem. Eng. J. 1988, 38, 149–152. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Tudose, R.Z. Effects of downcomer-to-riser cross sectional area ratio on operation behaviour of external-loop airlift bioreactors. Bioprocess Eng. 1996, 15, 77–85. [Google Scholar] [CrossRef]
- Basha, O.M.; Sehabiague, L.; Abdel-Wahab, A.; Morsi, B.I. Fischer–Tropsch Synthesis in Slurry Bubble Column Reactors: Experimental Investigations and Modeling—A Review. Int. J. Chem. React. Eng. 2015, 13, 201–288. [Google Scholar] [CrossRef]
- Zimmerman, W.H.; Bukur, D.B. Reaction kinetics over iron catalysts used for the fischer-tropsch synthesis. Can. J. Chem. Eng. 1990, 68, 292–301. [Google Scholar] [CrossRef]
- Hazare, S.R.; Vala, S.V.; Patil, C.S.; Joshi, A.J.; Joshi, J.B.; Vitankar, V.S.; Patwardhan, A.W. Correlating Interfacial Area and Volumetric Mass Transfer Coefficient in Bubble Column with the Help of Machine Learning Methods. Ind. Eng. Chem. Res. 2023, 62, 2104–2123. [Google Scholar] [CrossRef]










| Model | EXT | MLP | ||
|---|---|---|---|---|
| Training | Testing | Training | Testing | |
| MAPE (%) | 1.1 | 4.5 | 3.4 | 5.3 |
| R2 | 0.99 | 0.95 | 0.96 | 0.93 |
| HP tuning (s) | <60 | >9000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asbahr, W.; Lamparter, R.; Rauch, R. A Cold Flow Model of Interconnected Slurry Bubble Columns for Sorption-Enhanced Fischer–Tropsch Synthesis. ChemEngineering 2024, 8, 52. https://doi.org/10.3390/chemengineering8030052
Asbahr W, Lamparter R, Rauch R. A Cold Flow Model of Interconnected Slurry Bubble Columns for Sorption-Enhanced Fischer–Tropsch Synthesis. ChemEngineering. 2024; 8(3):52. https://doi.org/10.3390/chemengineering8030052
Chicago/Turabian StyleAsbahr, Wiebke, Robin Lamparter, and Reinhard Rauch. 2024. "A Cold Flow Model of Interconnected Slurry Bubble Columns for Sorption-Enhanced Fischer–Tropsch Synthesis" ChemEngineering 8, no. 3: 52. https://doi.org/10.3390/chemengineering8030052






