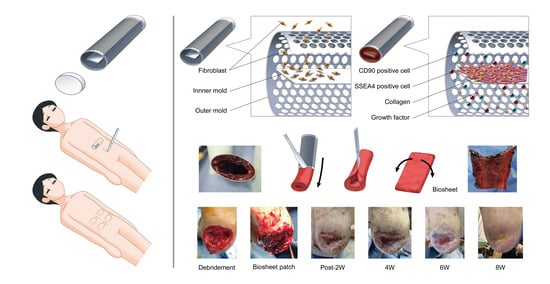
Dramatic Wound Closing Effect of a Single Application of an iBTA-Induced Autologous Biosheet on Severe Diabetic Foot Ulcers Involving the Heel Area
Abstract
:1. Introduction
2. Cases
3. Results
4. Discussion
5. Limitations and Future
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.X.; Branco, B.C.; Armstrong, D.G.; Mills, J.L., Sr. The society for vascular surgery lower extremity threatened limb classification system based on wound, ischemia, and foot infection (WIfI) correlates with risk of major amputation and time wound healing. J. Vasc. Surg. 2015, 61, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Zimmy, S.; Schatz, H.; Pfohl, M. Determinants and estimation of healing times in diabetic foot ulcers. J Diabtes Complicat. 2002, 16, 327–332. [Google Scholar]
- Nakayama, Y.; Ishibashi-Ueda, H.; Takamizawa, K. In vivo tissue-engineered small-caliber arterial graft prosthesis consisting of autologous tissue (Biotube). Cell Transplant. 2004, 13, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, N.; Mizuno, T.; Iwai, R.; Uechi, M.; Nakayama, Y. In-body tissue-engineered collagenous connective tissue membranes (BIOSHEETs) for potential corneal stromal substitution. J. Tissue Eng. Regen. Med. 2013, 10, E518–E526. [Google Scholar] [CrossRef] [PubMed]
- Furukoshi, M.; Moriwaki, T.; Nakayama, Y. Development of an in vivo tissue-engineered vascular graft with designed wall thickness (biotube type C) based on a novel caged mold. J. Artif. Organs 2016, 19, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Satake, R.; Komura, M.; Komura, H.; Kodaka, T.; Terawaki, K.; Ikebukuro, K.; Komuro, H.; Yonekawa, H.; Hoshi, K.; Takato, T.; et al. Patch tracheoplasty in body tissue engineering using collagenous connective tissue membranes (biosheets). J. Pediatr. Surg. 2016, 51, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Carsten, C.G., 3rd; Taylor, S.M.; Langan, E.M., 3rd; Crane, M.M. Factors associated with limb loss despite a patient infrainguinal bypass graft. Am. Surg. 1998, 64, 33–37, discussion 37–38. [Google Scholar] [PubMed]
- Edwards, J.M.; Taylor, L.M., Jr.; Porter, J.M. Limb salvage in end-stage renal disease (ESRD). Comparison of modern results in patients with and without ESRD. Arch. Surg. 1998, 123, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, M.; Aho, P.S.; Lepnatalo, M.; Albäck, A. The influence of the characteristics of ischemic tissue lesion on ulcer healing time after infrainguinal bypass for critical leg ischemia. J. Vasc. Surg. 2009, 49, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Hirano, K.; Nakano, M.; Muramatsu, T.; Tsukahara, R.; Ito, Y.; Ishimori, H. Wound healing and wound location in critical limb ischemia following endovascular treatment. Circ. J. 2014, 78, 1746–1753. [Google Scholar] [CrossRef]
- Sato, Y.; Iwai, R.; Fukushima, M.; Nakayama, Y. Involvement of somatic cells in encapsulation of foreign-body reaction in canine subcutaneous Biotube tissue formation. J. Biosci. Bioeng. 2021, 135, 524–530. [Google Scholar] [CrossRef]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L.; Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R.; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Zelen, C.M.; Serena, T.E.; Denoziere, G.; Fetterolf, D.E. A prospective randomized comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int. Wound J. 2013, 10, 502–507. [Google Scholar] [CrossRef]
- Fetterolf, D.E.; Istwan, N.B.; Stanziano, G.J. An evaluation of healing metrics associated with commonly used advanced wound care products for treatment of chronic foot ulcers. Manag. Care 2014, 23, 31–38. [Google Scholar] [PubMed]
- Zelen, C.M.; Gould, L.; Serena, T.E.; Carter, M.; Keller, J.; Li, W. A prospective, randomized, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int. Wound J. 2015, 12, 724–732. [Google Scholar] [PubMed]
- Tiscar-Gonzalez, V.; Menor-Rodriguez, M.J.; Rabadan-Sainz, C.; Fraile-Bravo, M.; Styche, T.; Valenzuela-Ocaña, F.J.; Muñoz-García, L. Clinical and economic impact of wound care using a polyurethane foam multilayer dressing. Adv. Skin Wound Care 2021, 10, 13–23. [Google Scholar] [CrossRef]







| Case | Age | Gender | Responsible Disease | Significant Medical Conditions | Revascularization | Additive Wound Treatment | Results (Dulation) |
|---|---|---|---|---|---|---|---|
| 1 | 46 | M | Diabetic neuropathy | DM, Obesity, HTN, DL | NPWT | healed (2M) | |
| 2 | 93 | M | Diabetic microvasculopathy | DM, CKDG5D, CI, HTN, AF | death with IE | ||
| 3 | 73 | M | CLTI (PAD) | DM, CKDG5D, CAD, HTN | EVT | SCS | healed (2M) |
| 4 | 53 | M | CLTI (PAD) | DM, CKDG5D, CAD, CI | EVT | NPWT | healed (5M) |
| 5 | 57 | M | Diabetic neuropathy | DM, CKDG5D, CAD | LDL apheresis | BKA | |
| 6 | 74 | M | CLTI (PAD) | DM, CKDG5D, CAD, AS(AVR), CI, HTN | EVT | LDL apheresis, PRP | healed(5M) |
| 7 | 69 | F | CLTI (PAD) | DM, CKDG5D | OS | healed (9M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashita, R.; Nakayama, Y.; Miyazaki, M.; Yokawa, Y.; Iwai, R.; Funayama-Iwai, M. Dramatic Wound Closing Effect of a Single Application of an iBTA-Induced Autologous Biosheet on Severe Diabetic Foot Ulcers Involving the Heel Area. Bioengineering 2024, 11, 462. https://doi.org/10.3390/bioengineering11050462
Higashita R, Nakayama Y, Miyazaki M, Yokawa Y, Iwai R, Funayama-Iwai M. Dramatic Wound Closing Effect of a Single Application of an iBTA-Induced Autologous Biosheet on Severe Diabetic Foot Ulcers Involving the Heel Area. Bioengineering. 2024; 11(5):462. https://doi.org/10.3390/bioengineering11050462
Chicago/Turabian StyleHigashita, Ryuji, Yasuhide Nakayama, Manami Miyazaki, Yoko Yokawa, Ryosuke Iwai, and Marina Funayama-Iwai. 2024. "Dramatic Wound Closing Effect of a Single Application of an iBTA-Induced Autologous Biosheet on Severe Diabetic Foot Ulcers Involving the Heel Area" Bioengineering 11, no. 5: 462. https://doi.org/10.3390/bioengineering11050462