Design and Synthesis of P(AAm-co-NaAMPS)-Alginate-Xanthan Hydrogels and the Study of Their Mechanical and Rheological Properties in Artificial Vascular Graft Applications
Abstract
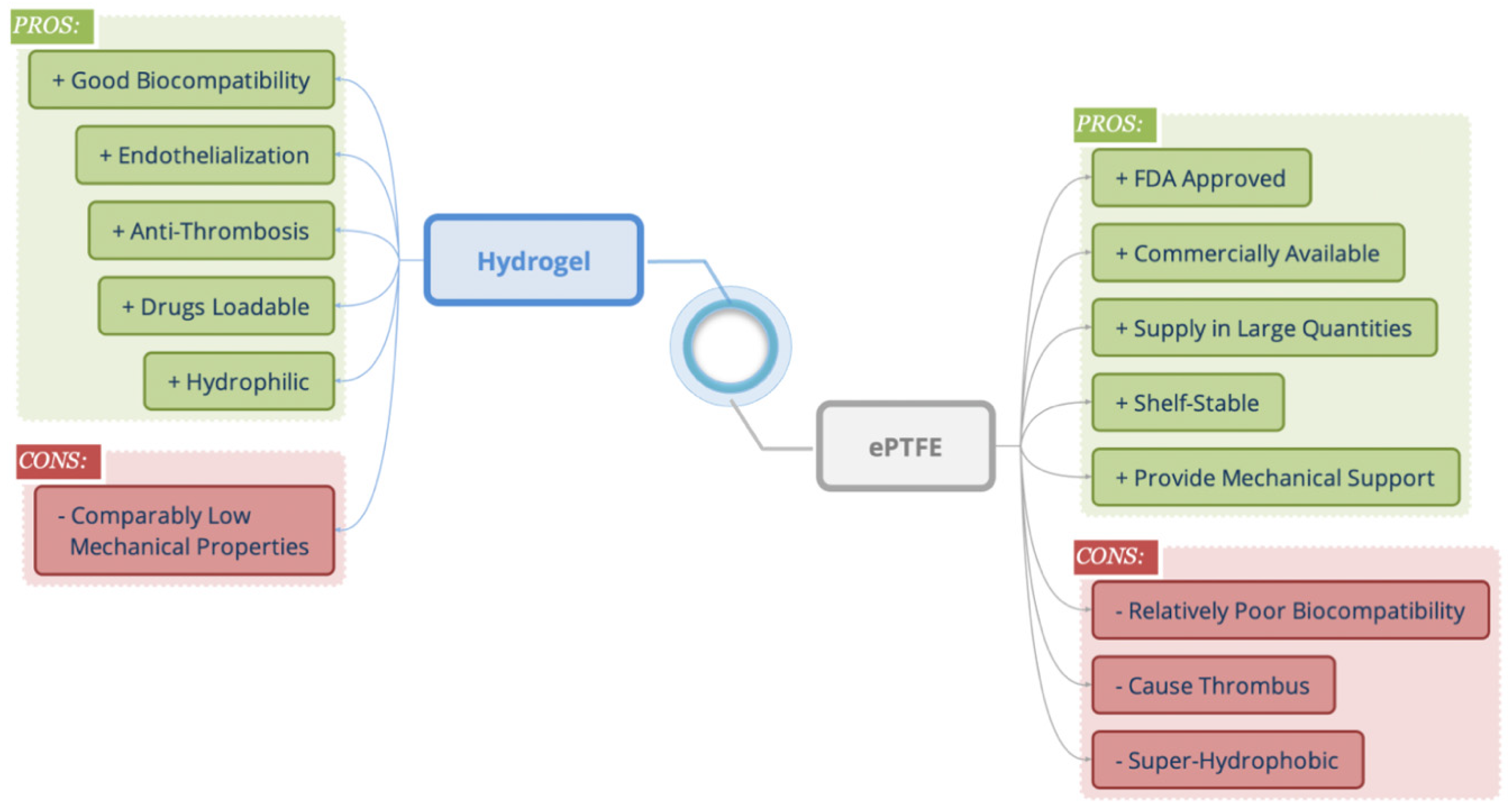
:1. Introduction
2. Results and Discussion
2.1. Physical Appearance and Microstructure of Hydrogels
2.2. Physical Appearance and Microstructure of ePTFE–Hydrogel (6A12M) Vascular Graft
2.3. FTIR Investigation of Hydrogels
2.4. Equilibrium Swelling Ratio of Hydrogels
2.5. Mechanical Properties of Hydrogels
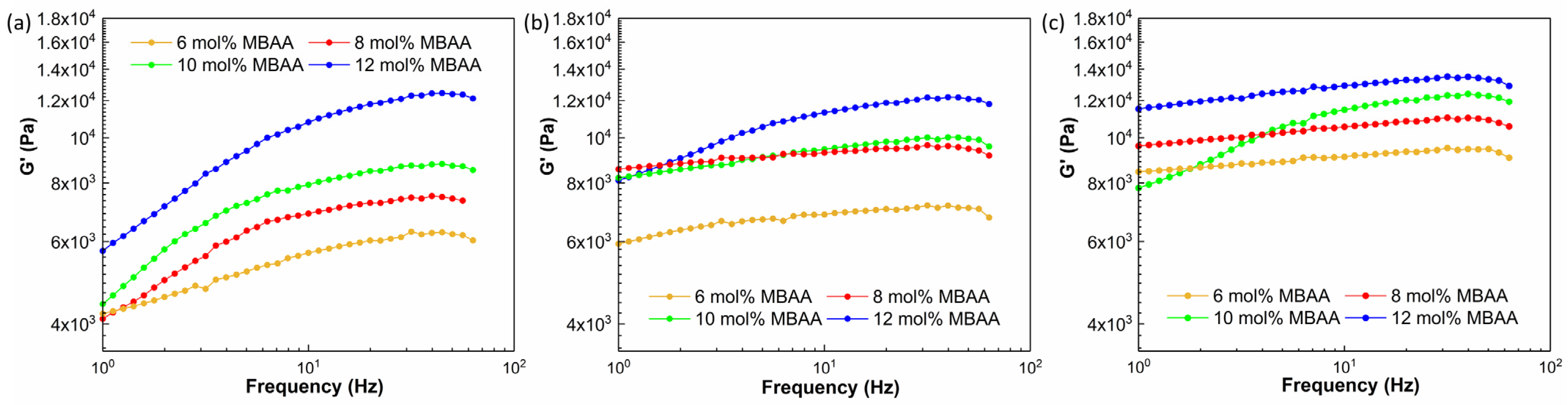
2.5.1. Rheology Test Results of Hydrogel Precursor Solutions
2.5.2. Rheology Test Results of Crosslinked Hydrogels
2.5.3. Cyclic Tensile Test Results of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Characterizations
4.3.1. Fourier Transform Infrared (FTIR) Analysis
4.3.2. Scanning Electron Microscopy (SEM) Analysis
4.3.3. Equilibrium Swelling Ratios
4.3.4. Mechanical Properties
Cyclic Tensile Test
Rheological Test
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaptoge, S.; Pennells, L.; De Bacquer, D.; Cooney, M.T.; Kavousi, M.; Stevens, G.; Riley, L.M.; Savin, S.; Khan, T.; Altay, S. World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob. Health 2019, 7, e1332–e1345. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Wang, Y.; Li, G.; Yang, L.; Luo, R.; Guo, G. Development of innovative biomaterials and devices for the treatment of cardiovascular diseases. Adv. Mater. 2022, 34, 2201971. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Maharjan, S.; Kuang, X.; Wang, Z.; Mille, L.S.; Tao, M.; Yu, P.; Cao, X.; Lian, L.; Lv, L. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci. Adv. 2022, 8, eabq6900. [Google Scholar] [CrossRef] [PubMed]
- Chlupáč, J.; Filova, E.; Bačáková, L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol. Res. 2009, 58, S119–S139. [Google Scholar] [CrossRef]
- Naegeli, K.M.; Kural, M.H.; Li, Y.; Wang, J.; Hugentobler, E.A.; Niklason, L.E. Bioengineering human tissues and the future of vascular replacement. Circ. Res. 2022, 131, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, C.H.; Kim, T.H.; Park, S.H. Soft Biomimetic 3D Free-Form Artificial Vascular Graft Using a Highly Uniform Microspherical Porous Structure. ACS Appl. Mater. Interfaces 2022, 14, 29588–29598. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Economides, C.; Mayeda, G.; Burstein, S.; Kloner, R. The endothelial cell in health and disease: Its function, dysfunction, measurement and therapy. Int. J. Impot. Res. 2010, 22, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Van Lith, R.; Jen, M.C.; Allen, J.B.; Lapidos, K.A.; Ameer, G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials 2013, 34, 30–41. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Li, Z.T.; Lin, Y.J.; Thomson, J.A.; Turng, L.S. Fabrication and modification of wavy multicomponent vascular grafts with biomimetic mechanical properties, antithrombogenicity, and enhanced endothelial cell affinity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2397–2408. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE functionalization for medical applications. Mater. Today Chem. 2021, 20, 100412. [Google Scholar] [CrossRef]
- Lavery, K.S.; Rhodes, C.; Mcgraw, A.; Eppihimer, M.J. Anti-thrombotic technologies for medical devices. Adv. Drug Deliv. Rev. 2017, 112, 2–11. [Google Scholar] [CrossRef]
- Fischer, P.E.; Schroeppel, T.J.; Fabian, T.C.; deRijk, W.G.; Edwards, N.M.; Magnotti, L.J.; Doty, D.H.; Croce, M.A. Antibiotic-coated ePTFE decreases graft colonization and neointimal hyperplasia. J. Surg. Res. 2009, 156, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Cassady, A.I.; Hidzir, N.M.; Grøndahl, L. Enhancing expanded poly (tetrafluoroethylene)(ePTFE) for biomaterials applications. J. Appl. Polym. Sci. 2014, 131, 40533. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Thomsom, J.A.; Turng, L.-S. Promoting endothelial cell affinity and antithrombogenicity of polytetrafluoroethylene (PTFE) by mussel-inspired modification and RGD/heparin grafting. J. Mater. Chem. B 2018, 6, 3475–3485. [Google Scholar] [CrossRef] [PubMed]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and modifications in the development of small-diameter vascular grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Al Meslmani, B.M.; Mahmoud, G.F.; Bakowsky, U. Development of expanded polytetrafluoroethylene cardiovascular graft platform based on immobilization of poly lactic-co-glycolic acid nanoparticles using a wet chemical modification technique. Int. J. Pharm. 2017, 529, 238–244. [Google Scholar] [CrossRef]
- Zhu, A.; Ming, Z.; Jian, S. Blood compatibility of chitosan/heparin complex surface modified ePTFE vascular graft. Appl. Surf. Sci. 2005, 241, 485–492. [Google Scholar] [CrossRef]
- Matsuura, S.; Takayama, T.; Oyama, T.G.; Oyama, K.; Taguchi, M.; Endo, T.; Akai, T.; Isaji, T.; Hoshina, K. A radiation-crosslinked gelatin hydrogel that promotes tissue incorporation of an expanded polytetrafluoroethylene vascular graft in rats. Biomolecules 2021, 11, 1105. [Google Scholar] [CrossRef]
- Zheng, W.J.; Chen, Q.; Zou, W.; Fu, Z.; Li, Y.; Liu, Z.; Yan, J.; Yang, H.; Yang, F. Waterproof and breathable wound dressing composited by expanded polytetrafluoroethylene backing and hydrogel. Macromol. Biosci. 2022, 22, 2200131. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, F.; Cai, P.; Wang, M.; He, K.; Luo, Y.; Li, Z.; Chen, G.; Ji, S.; Liu, Z. Mechanically interlocked hydrogel–elastomer hybrids for on-skin electronics. Adv. Funct. Mater. 2020, 30, 1909540. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Li, Z.; Giarto, J.; Zhang, J.; Kulkarni, N.; Mahalingam, E.; Klipstine, W.; Turng, L.-S. Anti-thrombotic poly (AAm-co-NaAMPS)-xanthan hydrogel-expanded polytetrafluoroethylene (ePTFE) vascular grafts with enhanced endothelialization and hemocompatibility properties. Biomater. Adv. 2023, 154, 213625. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2021, 6, 1791–1809. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Guan, G.; Glas, S.; Wang, L.; Li, Z.; Turng, L.-S. A biomimetic basement membrane consisted of hybrid aligned nanofibers and microfibers with immobilized collagen IV and laminin for rapid endothelialization. Bio-Des. Manuf. 2021, 4, 171–189. [Google Scholar] [CrossRef]
- Zhao, X. Designing toughness and strength for soft materials. Proc. Natl. Acad. Sci. USA 2017, 114, 8138–8140. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Arıcan, M.O.; Zhou, T.; Zhao, X.; Zhang, Y.S. Functional tough hydrogels: Design, processing, and biomedical applications. Acc. Mater. Res. 2022, 4, 101–114. [Google Scholar] [CrossRef]
- Luo, Y.; Pauer, W.; Luinstra, G.A. Tough, Stretchable, and Thermoresponsive Smart Hydrogels. Gels 2023, 9, 695. [Google Scholar] [CrossRef]
- Chen, Y.M.; Tanaka, M.; Gong, J.P.; Yasuda, K.; Yamamoto, S.; Shimomura, M.; Osada, Y. Platelet adhesion to human umbilical vein endothelial cells cultured on anionic hydrogel scaffolds. Biomaterials 2007, 28, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Shiraishi, N.; Satokawa, H.; Kakugo, A.; Narita, T.; Gong, J.P.; Osada, Y.; Yamamoto, K.; Ando, J. Cultivation of endothelial cells on adhesive protein-free synthetic polymer gels. Biomaterials 2005, 26, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Liu, Z.Q.; Xu, F.; Gao, J.; Chen, Y.M.; Gong, J.P.; Osada, Y. In Vitro Platelet Adhesion of PNaAMPS/PAAm and PNaAMPS/PDMAAm Double-Network Hydrogels. Macromol. Chem. Phys. 2015, 216, 641–649. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Iftime, M.M.; Ailiesei, G.-L.; Ipate, A.-M.; Bargan, A.; Vlad-Bubulac, T.; Rîmbu, C.M. Evaluation of Poly (vinyl alcohol)–Xanthan Gum Hydrogels Loaded with Neomycin Sulfate as Systems for Drug Delivery. Gels 2023, 9, 655. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Bueno, V.B.; Takahashi, S.H.; Catalani, L.H.; de Torresi, S.I.C.; Petri, D.F.S. Biocompatible xanthan/polypyrrole scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 52, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Cakić, S. A review of patents and innovative biopolymer-based hydrogels. Gels 2023, 9, 556. [Google Scholar] [CrossRef]
- Shan, J.; Guo, J.; Guan, F.; Li, F.; Di, C. Characteristics of Sodium Alginate/Antarctic Krill Protein Composite Fiber Based on Cellulose Nanocrystals Modification: Rheology, Hydrogen Bond, Crystallization, Strength, and Water-Resistance. Gels 2022, 8, 139. [Google Scholar] [CrossRef]
- Gialouri, A.; Saravanou, S.F.; Loukelis, K.; Chatzinikolaidou, M.; Pasparakis, G.; Bouropoulos, N. Thermoresponsive Alginate-Graft-pNIPAM/Methyl Cellulose 3D-Printed Scaffolds Promote Osteogenesis In Vitro. Gels 2023, 9, 984. [Google Scholar] [CrossRef]
- Valade, D.; Wong, L.K.; Jeon, Y.; Jia, Z.; Monteiro, M.J. Polyacrylamide hydrogel membranes with controlled pore sizes. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 129–138. [Google Scholar] [CrossRef]
- Hao, J.; Weiss, R. Viscoelastic and mechanical behavior of hydrophobically modified hydrogels. Macromolecules 2011, 44, 9390–9398. [Google Scholar] [CrossRef]
- Turng, L.-S.; Xu, Y.; Lin, Y.-J.; Wang, D.-F. Green Fabrication of Polytetrafluoroethylene and Expanded Polytetrafluoroethylene and Uses Thereof. U.S. Patent 11,339,267, 24 May 2022. [Google Scholar]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]

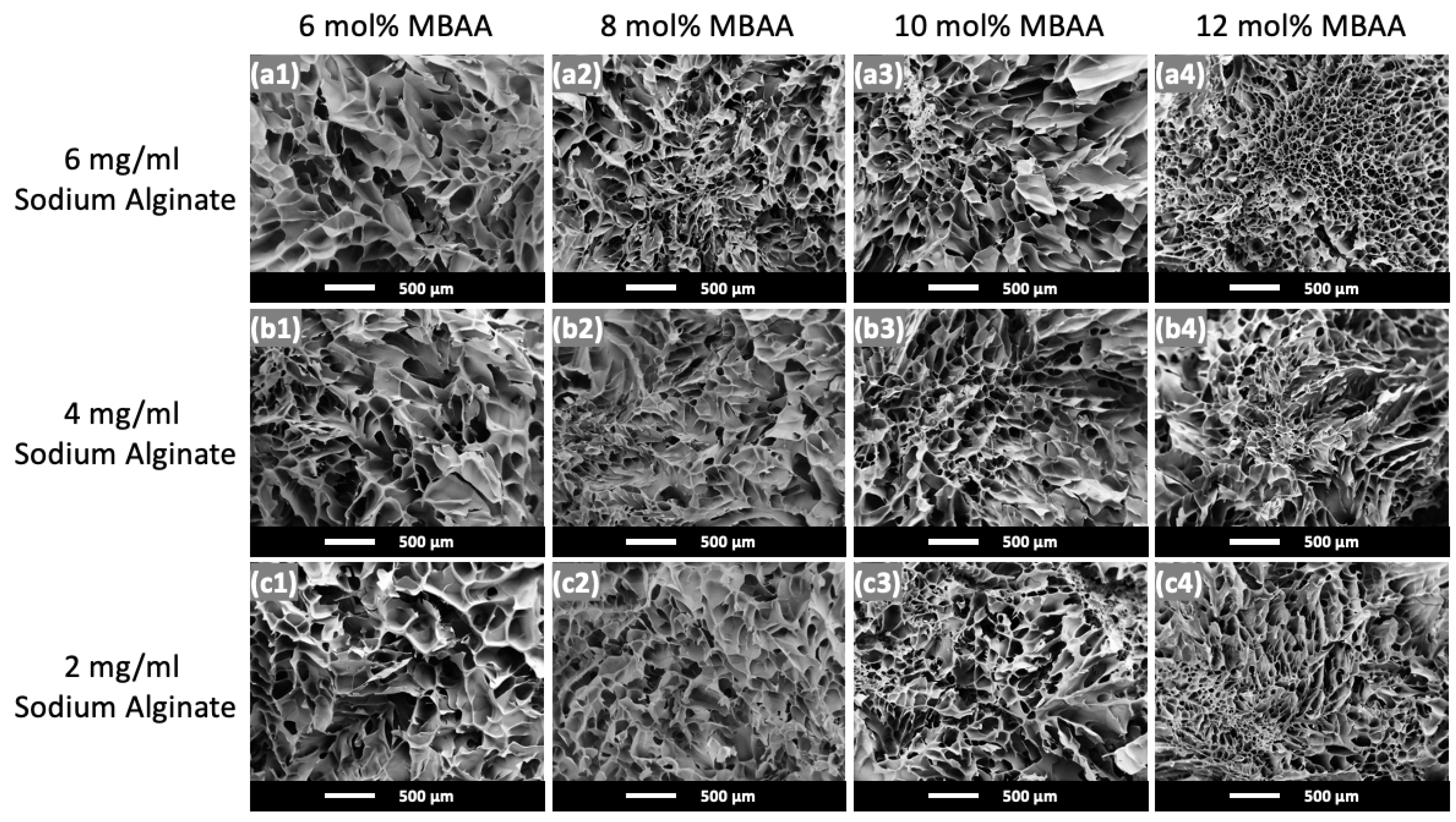









| Pore Diameter (μm) | ||||
|---|---|---|---|---|
| 6 mol% MBAA | 8 mol% MBAA | 10 mol% MBAA | 12 mol% MBAA | |
| 6 mg/mL Alginate | 316.81 ± 70.09 | 189.58 ± 44.93 | 235.49 ± 63.47 | 139.78 ± 24.85 |
| 4 mg/mL Alginate | 352.04 ± 86.36 | 261.03 ± 74.33 | 242.92 ± 42.37 | 205.08 ± 69.42 |
| 2 mg/mL Alginate | 330.47 ± 71.75 | 241.28 ± 58.92 | 203.95 ± 64.43 | 155.97 ± 20.13 |
| Hydrogel Type | Alginate (mg/mL) | MBAA (mol%) |
|---|---|---|
| Hydrogel (2A6M) | 2 | 6 |
| Hydrogel (2A8M) | 8 | |
| Hydrogel (2A10M) | 10 | |
| Hydrogel (2A12M) | 12 | |
| Hydrogel (4A6M) | 4 | 6 |
| Hydrogel (4A8M) | 8 | |
| Hydrogel (4A10M) | 10 | |
| Hydrogel (4A12M) | 12 | |
| Hydrogel (6A6M) | 6 | 6 |
| Hydrogel (6A8M) | 8 | |
| Hydrogel (6A10M) | 10 | |
| Hydrogel (6A12M) | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Giarto, J.; Zhang, J.; Gim, J.; Chen, E.; Enriquez, E.; Jafuta, L.; Mahalingam, E.; Turng, L.-S. Design and Synthesis of P(AAm-co-NaAMPS)-Alginate-Xanthan Hydrogels and the Study of Their Mechanical and Rheological Properties in Artificial Vascular Graft Applications. Gels 2024, 10, 319. https://doi.org/10.3390/gels10050319
Li Z, Giarto J, Zhang J, Gim J, Chen E, Enriquez E, Jafuta L, Mahalingam E, Turng L-S. Design and Synthesis of P(AAm-co-NaAMPS)-Alginate-Xanthan Hydrogels and the Study of Their Mechanical and Rheological Properties in Artificial Vascular Graft Applications. Gels. 2024; 10(5):319. https://doi.org/10.3390/gels10050319
Chicago/Turabian StyleLi, Zhutong, Joshua Giarto, Jue Zhang, Jinsu Gim, Edward Chen, Eduardo Enriquez, Lauren Jafuta, Esha Mahalingam, and Lih-Sheng Turng. 2024. "Design and Synthesis of P(AAm-co-NaAMPS)-Alginate-Xanthan Hydrogels and the Study of Their Mechanical and Rheological Properties in Artificial Vascular Graft Applications" Gels 10, no. 5: 319. https://doi.org/10.3390/gels10050319






