Innovative Acrylic Resin-Hydrogel Double-Layer Coating: Achieving Dual-Anchoring, Enhanced Adhesion, and Superior Anti-Biofouling Properties for Marine Applications
Abstract
:1. Introduction
2. Result and Discussion
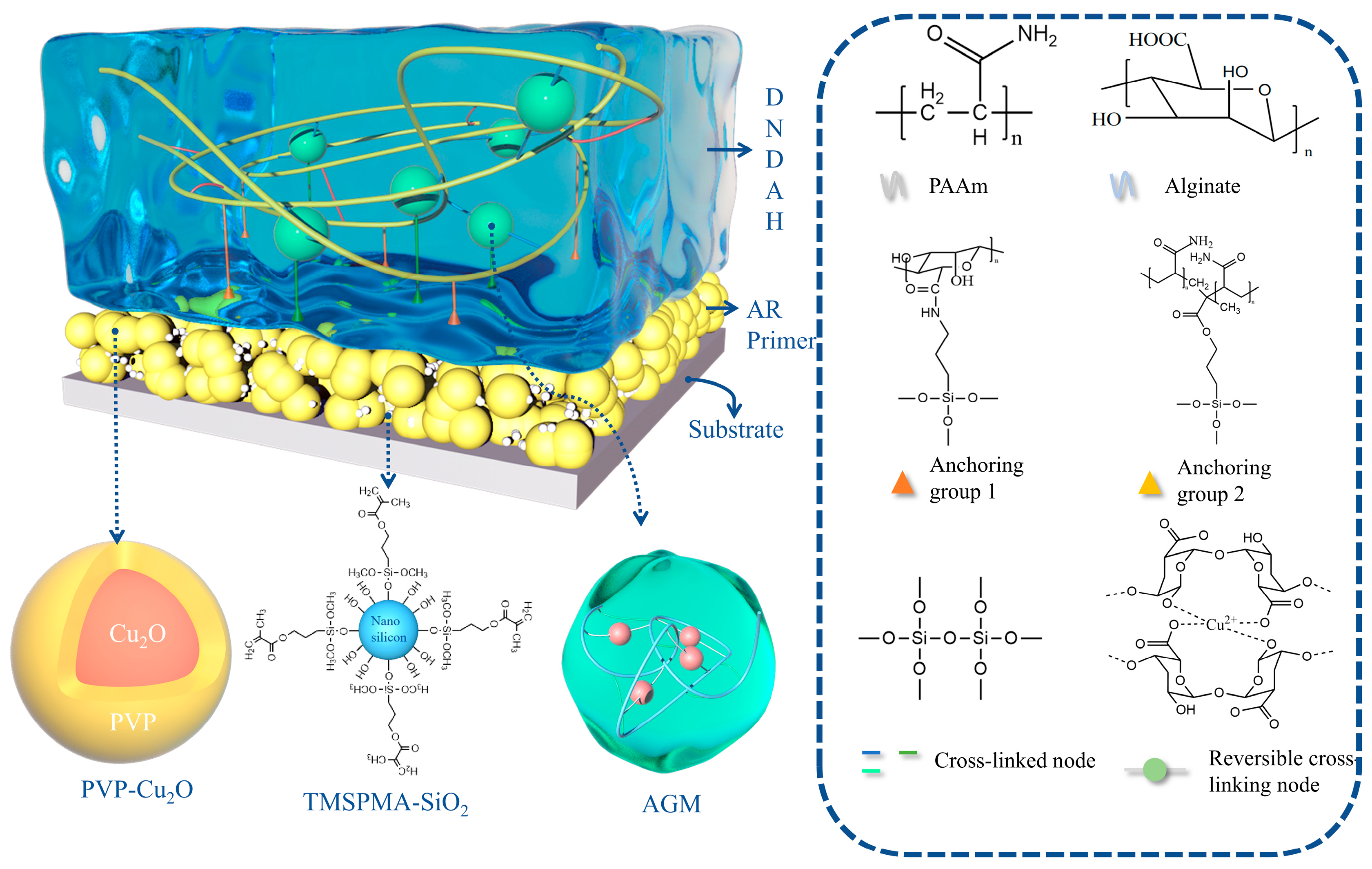
2.1. Construction of the A-H DL Coating System
2.2. Analysis of the Internal Chemical Structure of the Coatings
2.2.1. Internal Structure of DNDAH
2.2.2. Internal Structure of AR Primer
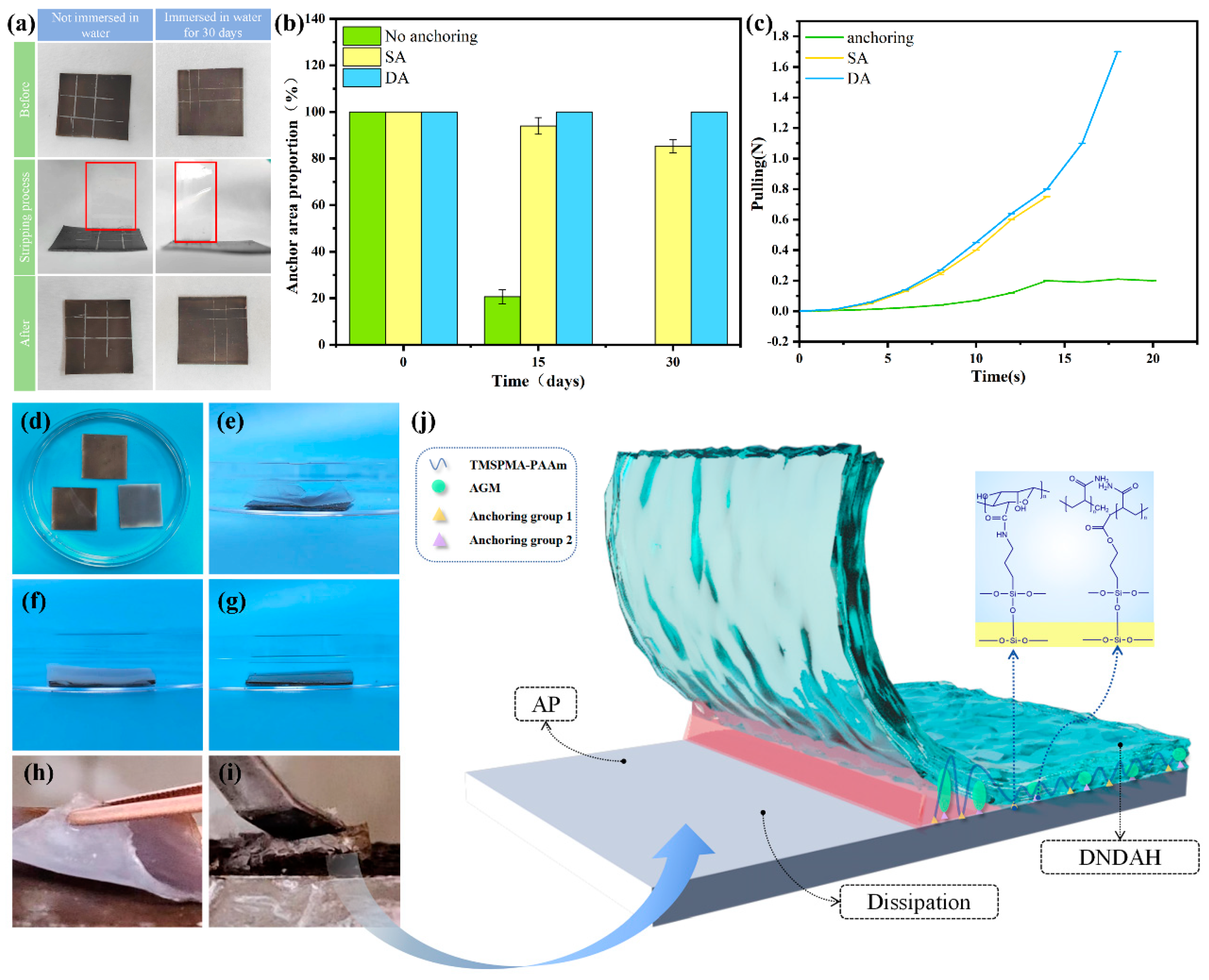
2.3. Anchoring Performance and Mechanical Properties of A-H DL Coating
2.3.1. Internal Tensile Strength of DNDAH
2.3.2. The Adhesion Properties of A-H DL
2.4. Functional Evaluation of the Coatings
2.4.1. Cu2+ Release Performance
2.4.2. Anti-Protein Adsorption Ability
2.4.3. Marine Simulation Experiment
2.5. Considerations about Monitoring and Maintenance Strategies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of AR Primer
4.3. Preparation of A-H DL
4.4. Characterization
4.5. Mechanical Performance Testing
4.6. Cu2+ Release and Functional Testing
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 2020, 148, 105821. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, X.; Zhang, H.; Li, W.; Jiang, R.; Zhou, X. Review on formation of biofouling in the marine environment and functionalization of new marine antifouling coatings. J. Mater. Sci. 2022, 57, 18221–18242. [Google Scholar] [CrossRef]
- Tian, L.; Yin, Y.; Jin, H.; Bing, W.; Jin, E.; Zhao, J.; Ren, L. Novel marine antifouling coatings inspired by corals. Mater. Today Chem. 2020, 17, 100294. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, L.; Mou, J.; Wu, D.; Xu, M.; Zhou, P.; Ren, Y. Research Strategies to Develop Environmentally Friendly Marine Antifouling Coatings. Mar. Drugs 2020, 18, 170371. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Williams, M.D.; Falkinham Iii, J.O.; Ducker, W.A. Antimicrobial mechanism of cuprous oxide (Cu2O) coatings. J. Colloid Interface Sci. 2023, 652, 1867–1877. [Google Scholar] [CrossRef]
- Liu, D.; Shu, H.; Zhou, J.; Bai, X.; Cao, P. Research Progress on New Environmentally Friendly Antifouling Coatings in Marine Settings: A Review. Biomimetics 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Jalaie, A.; Afshaar, A.; Mousavi, S.B.; Heidari, M. Investigation of the Release Rate of Biocide and Corrosion Resistance of Vinyl-, Acrylic-, and Epoxy-Based Antifouling Paints on Steel in Marine Infrastructures. Polymers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Carrier, A.J.; Carve, M.; Shimeta, J.; Walker, T.R.; Zhang, X.; Oakes, K.D.; Jha, K.C.; Charlton, T.; Stenzel, M.H. Transitioning towards environmentally benign marine antifouling coatings. Front. Mar. Sci. 2023, 10, 1175270. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef]
- Ovari, T.-R.; Szőke, Á.F.; Katona, G.; Szabó, G.S.; Muresan, L.M. Temporary Anti-Corrosive Double Layer on Zinc Substrate Based on Chitosan Hydrogel and Epoxy Resin. Gels 2023, 9, 361. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, 39. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, C. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Eritja, R.; Díaz Díaz, D. On the Race for More Stretchable and Tough Hydrogels. Gels 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ao, Y.; Lin, T.; Yang, X.; Peng, J.; Huang, W.; Li, J.; Zhai, M. High-toughness polyacrylamide gel containing hydrophobic crosslinking and its double network gel. Polymer 2016, 87, 73–80. [Google Scholar] [CrossRef]
- Narasimhan, B.N.; Deijs, G.S.; Manuguri, S.; Ting, M.S.H.; Williams, M.A.K.; Malmström, J. A comparative study of tough hydrogen bonding dissipating hydrogels made with different network structures. Nanoscale Adv. 2021, 3, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogelsviahybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Liu, Y.; Liu, W.; Dong, L.; Yu, L.; Wang, L. A highly antifouling and eco-friendly hydrogel coating based on capsaicin derivative -functionalized polymer. J. Clean. Prod. 2023, 429, 139538. [Google Scholar] [CrossRef]
- Li, X.; Gou, J.; Feng, H.; Sun, X.; Qin, X.; Wang, W.; Li, W.; Chen, S. Improved Antifouling Ability for Double-Network Hydrogel Coatings with Excellent Elastic and Toughness under Marine Tidal Environment. Adv. Eng. Mater. 2023, 25, 2201801. [Google Scholar] [CrossRef]
- Takahashi, R.; Shimano, K.; Okazaki, H.; Kurokawa, T.; Nakajima, T.; Nonoyama, T.; King, D.R.; Gong, J.P. Tough Particle-Based Double Network Hydrogels for Functional Solid Surface Coatings. Adv. Mater. Interfaces 2018, 5, 1801018. [Google Scholar] [CrossRef]
- Yang, J.; Xue, B.; Zhou, Y.; Qin, M.; Wang, W.; Cao, Y. Spray-Painted Hydrogel Coating for Marine Antifouling. Adv. Mater. Technol. 2021, 6, 200911. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Feng, D.-Q.; Zhu, P.-Y.; Song, W.-L.; Yasir, M.; Zhang, C.; Liu, L. Hydrogel-Anchored Fe-Based Amorphous Coatings with Integrated Antifouling and Anticorrosion Functionality. ACS Appl. Mater. Interfaces 2023, 15, 13644–13655. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2015, 15, 190–196. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 190–196. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat. Commun. 2020, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Tegelström, H.; Wyöni, P.I. Silanization of supporting glass plates avoiding fixation of polyacrylamide gels to glass cover plates. Electrophoresis 2005, 7, 99. [Google Scholar] [CrossRef]
- Nakamura, S.; Luna, J.A.; Kakiuchida, H.; Hozumi, A. Effective Approach to Render Stable Dynamic Omniphobicity and Icephobicity to Ultrasmooth Metal Surfaces. Langmuir 2021, 37, 11771–11780. [Google Scholar] [CrossRef]
- Simunin, M.M.; Voronin, A.S.; Fadeev, Y.V.; Mikhlin, Y.L.; Lizunov, D.A.; Samoilo, A.S.; Chirkov, D.Y.; Voronina, S.Y.; Khartov, S.V. Features of Functionalization of the Surface of Alumina Nanofibers by Hydrolysis of Organosilanes on Surface Hydroxyl Groups. Polymers 2021, 13, 4374. [Google Scholar] [CrossRef]
- Prakash, I.; Muralidharan, P.; Nallamuthu, N.; Venkateswarlu, M.; Satyanarayana, N. Preparation and characterization of nanocrystallite size cuprous oxide. Mater. Res. Bull. 2007, 42, 1619–1624. [Google Scholar] [CrossRef]
- Mireles, L.K.; Wu, M.-R.; Saadeh, N.; Yahia, L.H.; Sacher, E. Physicochemical Characterization of Polyvinyl Pyrrolidone: A Tale of Two Polyvinyl Pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Chen, S.; Liu, X.; Yu, Y.; Li, T.; Wan, Y.; Tang, N.; Liu, Y.; Gu, Y. Bioinspired marine antifouling coatings: Antifouling mechanisms, design strategies and application feasibility studies. Eur. Polym. J. 2023, 190, 111997. [Google Scholar] [CrossRef]
- Petelinšek, N.; Mommer, S. Tough Hydrogels for Load-Bearing Applications. Adv. Sci. 2024, 681, 2307404. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yang, Q.; Tang, J.; Morelle, X.P.; Vlassak, J.; Suo, Z. Fatigue fracture of tough hydrogels. Extrem. Mech. Lett. 2017, 15, 91–96. [Google Scholar] [CrossRef]
- Mao, T.; Lu, G.; Xu, C.; Yu, H.; Yu, J. Preparation and properties of polyvinylpyrrolidone-cuprous oxide microcapsule antifouling coating. Prog. Org. Coat. 2020, 141, 105317. [Google Scholar] [CrossRef]
- Goswami, R.; Gogoi, M.; Borah, A.; Sarmah, H.; Borah, A.R.; Feng, X.; Hazarika, S. Quantum dot- β-Cyclodextrin nanofiller decorated thin film nanocomposite membrane for removal of cationic and anionic dyes from aqueous solution. Mater. Today Chem. 2024, 35, 101871. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Man, J.; Qu, Y.; Guo, Z.; Zhang, S.; Li, D. Mechanism of anti-proteins adsorption behavior on superhydrophobic titanium surface. Surf. Coat. Technol. 2021, 421, 127421. [Google Scholar] [CrossRef]
- Arya, R.K.; Verros, D.; Davim, J.P. Functional Coatings: Innovations and Challenges; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 110–152. [Google Scholar]
- Bora, P.; Bhuyan, C.; Borah, A.R.; Hazarika, S. Carbon Nanomaterials for Designing Next-generation Membranes and Their Emerging Applications. Chem. Commun. 2023, 59, 11320–11336. [Google Scholar] [CrossRef]
- Karnati, S.R.; Oldham, D.; Fini, E.H.; Zhang, L. Application of surface-modified silica nanoparticles with dual silane coupling agents in bitumen for performance enhancement. Constr. Build. Mater. 2020, 244, 118324. [Google Scholar] [CrossRef]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M. Preparation and characterization of acrylic resins with bioactive glasses. Sci. Rep. 2022, 12, 16624. [Google Scholar] [CrossRef]
- Chu, H.-H.; Chang, C.-Y.; Shen, B.-H. An electrophoretic coating using a nanosilica modified polyacrylate resin. J. Polym. Res. 2018, 25, 44. [Google Scholar] [CrossRef]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A. Characterization and Applications of Nanoparticles Modified in-Flight with Silica or Silica-Organic Coatings. Nanomaterials 2018, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Amerio, E.; Fabbri, P.; Malucelli, G.; Messori, M.; Sangermano, M.; Taurino, R. Scratch resistance of nano-silica reinforced acrylic coatings. Prog. Org. Coat. 2008, 62, 129–133. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Zhao, W.; Xie, C.; Zhu, Y.; Wen, C.; Li, Q.; Sui, X.; Yang, J.; Zhang, L. A polyvinylpyrrolidone-based surface-active copolymer for an effective marine antifouling coating. Prog. Org. Coat. 2021, 150, 105975. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Fadaei Tehrani, A. Abrasion resistance of acrylic polyurethane coatings reinforced by nano-silica. Prog. Org. Coat. 2018, 125, 507–515. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, S.; Lin, D.; Zhang, Z.; Li, G. Functional anti-corrosive and anti-bacterial surface coatings based on cuprous oxide/polyaniline microcomposites. Mater. Des. 2022, 216, 110589. [Google Scholar] [CrossRef]
- Hosoya, K.; Ohtsuki, C.; Kawai, T.; Kamitakahara, M.; Ogata, S.; Miyazaki, T.; Tanihara, M. A novel covalently crosslinked gel of alginate and silane with the ability to form bone-like apatite. J. Biomed. Mater. Res. Part A 2004, 71A, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Asefnejad, A.; Daliri Joupari, M.; Joughehdoust, S. The physicochemical and mechanical investigation of siloxane modified Gelatin/Sodium alginate injectable hydrogels loaded by ascorbic acid and β-Glycerophosphate. Mater. Today Commun. 2021, 26, 101914. [Google Scholar] [CrossRef]
- Hao, D.; Wang, Z.; Liu, M.; Guo, X.; Wang, S.; Jiang, L. Strong Anchoring of Hydrogels through Superwetting-Assisted High-Density Interfacial Grafting. Angew. Chem. Int. Ed. 2022, 62, e202215034. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Chen, S.; Wang, B.; Liu, S.; Cui, E.; Li, F.; Yu, Y.; Pan, W.; Tang, N.; et al. Polyvinyl Alcoholchitosan Based Nanocomposite Prganohydrogel Flexible. Int. J. Biol. Macromol. 2024, 258, 129054. [Google Scholar] [CrossRef]
- Qu, G.; Li, Y.; Yu, Y.; Huang, Y.; Zhang, W.; Zhang, H.; Liu, Z.; Kong, T. Spontaneously Regenerative Tough Hydrogels. Angew. Chem. Int. Ed. 2019, 58, 10951–10955. [Google Scholar] [CrossRef]
- Neha, R.; Thakare; Pravin, G.; Ingole; Hazarika, S. Biogenic Synthesis of Nanoparticles from the Edible Plant Polygonum microcephalum for Use in Antimicrobial Fabric. ACS Omega 2023, 8, 45301–45312. [Google Scholar]
- Jiang, L.; Jiang, B.; Xu, J.; Wang, T. Preparation of pH-responsive oxidized regenerated cellulose hydrogels compounded with nano-ZnO/chitosan/aminocyclodextrin ibuprofen complex for wound dressing. Int. J. Biol. Macromol. 2023, 253, 126628. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Parasuraman, V.R.; Mekuria, S.L.; Peng, S.; Tsai, H.-C.; Hsiue, G.-H. Plasma initiated graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on silicone elastomer surfaces to enhance bio(hemo)compatibility. Surf. Coat. Technol. 2017, 315, 342–349. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Mielczarski, E.; Rai, B.; Mielczarski, J.A. Quantitative and Qualitative Evaluation of Adsorption/Desorption of Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces. Langmuir 2009, 25, 11614–11620. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 136–138. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.; Chen, D. Influence of Illumination on Settlement of Diatom Navicula sp. Microb. Ecol. 2011, 62, 931–940. [Google Scholar] [CrossRef]
- Wickmann, C.; Sander, M. Influence of artificial seawater on the VHCF behavior of different steels. Fatigue Fract. Eng. Mater. Struct. 2022, 46, 715–727. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Q.; Xu, J.; Wang, T. Development of a Multifunctional Composite Hydrogel for Enhanced Wound Healing: Hemostasis, Sterilization, and Long-Term Moisturizing Properties. ACS Appl. Mater. Interfaces 2024, 16, 2972–2983. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Zhang, Y.; Wang, R.; Wang, T.; Zeng, E. Innovative Acrylic Resin-Hydrogel Double-Layer Coating: Achieving Dual-Anchoring, Enhanced Adhesion, and Superior Anti-Biofouling Properties for Marine Applications. Gels 2024, 10, 320. https://doi.org/10.3390/gels10050320
Jiang B, Zhang Y, Wang R, Wang T, Zeng E. Innovative Acrylic Resin-Hydrogel Double-Layer Coating: Achieving Dual-Anchoring, Enhanced Adhesion, and Superior Anti-Biofouling Properties for Marine Applications. Gels. 2024; 10(5):320. https://doi.org/10.3390/gels10050320
Chicago/Turabian StyleJiang, Boning, Yuhan Zhang, Ruiyang Wang, Ting Wang, and En Zeng. 2024. "Innovative Acrylic Resin-Hydrogel Double-Layer Coating: Achieving Dual-Anchoring, Enhanced Adhesion, and Superior Anti-Biofouling Properties for Marine Applications" Gels 10, no. 5: 320. https://doi.org/10.3390/gels10050320




