Machine Learning in Allergic Contact Dermatitis: Identifying (Dis)similarities between Polysensitized and Monosensitized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Polysensitization
2.3. MOAHLFA Index
2.4. Data Analysis
3. Results
3.1. Patients’ Demographic and Clinical Characteristics
3.2. Polysensitization
3.3. Associations among Patients’ Characteristics
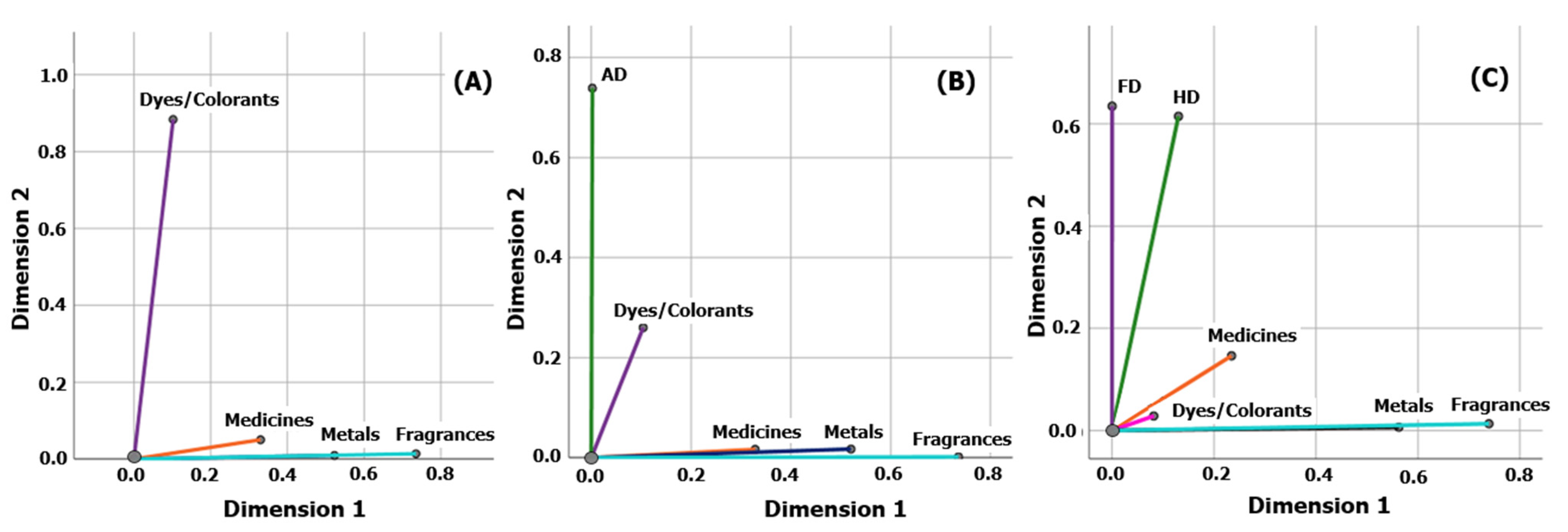
3.4. Multiple Correspondence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brites, G.S.; Ferreira, I.; Sebastião, A.I.; Silva, A.; Carrascal, M.; Neves, B.M.; Cruz, M. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies. Pharmacol. Res. 2020, 162, 105282. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, C.B.; Andersen, E.K.; Menne, T.; Johansen, D.J. Patients with multiple contact allergies: A review. Contact Dermat. 2008, 58, 1–8. [Google Scholar] [CrossRef]
- Schnuch, A.; Brasch, J.; Uter, W. Polysensitization and increased susceptibility in contact allergy: A review. Allergy 2008, 63, 156–167. [Google Scholar] [CrossRef]
- Gosnell, L.A.; Schmotzer, B.; Nedorost, T.S. Polysensitization and Individual Susceptibility to Allergic Contact Dermatitis. Contact Dermat. 2015, 26, 133–135. [Google Scholar] [CrossRef]
- Dittmar, D.; Uter, W.; Bauer, A.; Fortina, A.B.; Bircher, A.J.; Czarnecka-Operacz, M.; Dugonik, A.; Elsner, P.; Gallo, R.; Ghaffar, S.A.; et al. European Surveillance System on Contact Allergies (ESSCA): Polysensitization, 2009–2014. Contact Dermat. 2018, 78, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Bauer, A.; Fortina, A.B.; Bircher, A.J.; Brans, R.; Buhl, T.; Cooper, S.M.; Czarnecka-Operacz, M.; Dickel, H.; Dugonik, A.; et al. Patch test results with the European baseline series and additions thereof in the ESSCA network, 2015–2018. Contact Dermat. 2021, 84, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Tagka, A.; Stratigos, A.; Stavropoulos, P.; Rigopoulos, D.; Chatziioannou, A. An epidemiological study of allergic contact dermatitis in Greece: Prevalence of sensitization to an adapted European baseline series allergens. Int. J. Res. Dermatol. 2018, 4, 460–470. [Google Scholar] [CrossRef]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Yiannias, J. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allergy Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef]
- Rocha, V.B.; Scherrer, M.A.R. Thimerosal: Current sources of contact in Brazil. An. Bras. Dermatol. 2014, 89, 376–378. [Google Scholar] [CrossRef]
- Frosch, J.P.; Kugler, K. Contact Dermatitis, 5th ed.; Johansen, D.J., Frosch, J.P., Lepoittevin, P.J., Eds.; Springer: New York, NY, USA, 2010; pp. 831–840. [Google Scholar]
- Ibler, S.K.; Jemec, G.; Garvey, L.; Agner, T. Prevalence of delayed-type and immediate-type hypersensitivity in healthcare workers with hand eczema. Contact Dermat. 2016, 75, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Karalis, V. The Integration of Artificial Intelligence into Clinical Practice. Appl. Biosci. 2024, 3, 14–44. [Google Scholar] [CrossRef]
- Chan, W.; Srivastava, R.; Damaraju, N.; Do, H.; Burnett, G.; MacFarlane, J.; Xie, S.; Chen, J.; Honari, G.; Sarin, K. Automated detection of skin reactions in epicutaneous patch testing using machine learning. Br. J. Dermatol. 2021, 185, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, K.; Tagka, A.; Vezakis, I.; Kakkos, I.; Kyritsi, A.; Matsopoulos, G. Allergic Contact Dermatitis Detection with Machine Learning. TechRxiv 2024, preprints. [Google Scholar] [CrossRef]
- Greenacre, M.; Blasius, J. Multiple Correspondence Analysis and Related Methods, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2006. [Google Scholar]
- Shai, S.; Shai, B.D. Understanding Machine Learning: From Theory to Algorithms, 1st ed.; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Newell, L.; Polak, M.E.; Perera, J.; Owen, C.; Boyd, P.; Pickard, C.; Howarth, P.H.; Healy, E.; Holloway, J.W.; Friedmann, P.S.; et al. Sensitization via healthy skin programs Th2 responses in individuals with atopic dermatitis. J. Investig. Dermatol. 2013, 133, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Schnuch, A.; Uter, W.; Reich, K. Allergic contact dermatitis and atopic eczema. In Handbook of Atopic Eczema, 2nd ed.; Ring, J., Przybilla, B., Ruzicka, T., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; Chapter 17; pp. 176–199. [Google Scholar]
- Oosterhaven, J.; Uter, W.; Aberer, W.; Armario-Hita, J.C.; Ballmer-Weber, B.K.; Bauer, A.; Czarnecka-Operacz, M.; Elsner, P.; García-Gavín, J.; Giménez-Arnau, A.M.; et al. European Surveillance System on Contact Allergies (ESSCA): Contact allergies in relation to body sites in patients with allergic contact dermatitis. Contact Dermat. 2019, 80, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Requa, J.; Dao, T.; Ninh, A.; Tran, E.; Mai, D.; Lugo, M.; El-Hage Chehade, N.; Chang, K.J.; Karnes, W.E.; et al. Artificial Intelligence Using Convolutional Neural Networks for Real-Time Detection of Early Esophageal Neoplasia in Barrett’s Esophagus (with Video). Gastrointest. Endosc. 2020, 91, 1264–1271.e1. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for Cardiac Contractile Dysfunction Using an Artificial Intelligence–Enabled Electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Safaee Fakhr, B.; Ieppariello, G.; Foletto, M. Perioperative Care of the Obese Patient. Br. J. Surg. 2020, 107, e39–e55. [Google Scholar] [CrossRef]
- Attallah, O.; Sharkas, M.A.; Gadelkarim, H. Fetal Brain Abnormality Classification from MRI Images of Different Gestational Age. Brain Sci. 2019, 9, 231. [Google Scholar] [CrossRef]
- Xue, B.; Li, D.; Lu, C.; King, C.R.; Wildes, T.; Avidan, M.S.; Kannampallil, T.; Abraham, J. Use of Machine Learning to Develop and Evaluate Models Using Preoperative and Intraoperative Data to Identify Risks of Postoperative Complications. JAMA Netw. Open 2021, 4, e212240. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.O.; Pedreira, C.E.; Barrena, S.; Lopez, A.; Orfao, A. A Decision-Tree Approach for the Differential Diagnosis of Chronic Lymphoid Leukemias and Peripheral B-Cell Lymphomas. Comput. Methods Programs Biomed. 2019, 178, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, J.; Sun, Y.; Li, C.; Wu, W.; Jin, L.; Yang, Z.; Ni, B.; Gao, P.; Wang, P.; et al. 3D Deep Learning from CT Scans Predicts Tumor Invasiveness of Subcentimeter Pulmonary Adenocarcinomas. Cancer Res. 2018, 78, 6881–6889. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Grinband, J.; Weinberg, B.D.; Bardis, M.; Khy, M.; Cadena, G.; Su, M.-Y.; Cha, S.; Filippi, C.G.; Bota, D.; et al. Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. Am. J. Neuroradiol. 2018, 39, 1201–1207. [Google Scholar] [CrossRef]
- Freeman, K.; Dinnes, J.; Chuchu, N.; Takwoingi, Y.; Bayliss, S.E.; Matin, R.N.; Jain, A.; Walter, F.M.; Williams, H.C.; Deeks, J.J. Algorithm Based Smartphone Apps to Assess Risk of Skin Cancer in Adults: Systematic Review of Diagnostic Accuracy Studies. BMJ 2020, 368, m127. [Google Scholar] [CrossRef] [PubMed]
- Quinten, V.M.; van Meurs, M.; Wolffensperger, A.E.; ter Maaten, J.C.; Ligtenberg, J.J.M. Sepsis Patients in the Emergency Department. Eur. J. Emerg. Med. 2018, 25, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Boussard, C.; Bastard, P. Artificial Intelligence Can Predict GFR Decline During the Course of ADPKD. Am. J. Kidney Dis. 2018, 71, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, Y.; Sun, H. Intelligent Algorithm-Based Analysis on Ultrasound Image Characteristics of Patients with Lower Extremity Arteriosclerosis Occlusion and Its Correlation with Diabetic Mellitus Foot. J. Healthc. Eng. 2021, 2021, 7758206. [Google Scholar] [CrossRef]
- Cicione, A.; De Nunzio, C.; Manno, S.; Damiano, R.; Posti, A.; Lima, E.; Tubaro, A.; Balloni, F. An Update on Prostate Biopsy in the Era of Magnetic Resonance Imaging. Minerva Urol. Nephrol. 2018, 70, 264–274. [Google Scholar] [CrossRef]
- Peng, Y.; Dharssi, S.; Chen, Q.; Keenan, T.D.; Agrón, E.; Wong, W.T.; Chew, E.Y.; Lu, Z. DeepSeeNet: A Deep Learning Model for Automated Classification of Patient-Based Age-Related Macular Degeneration Severity from Color Fundus Photographs. Ophthalmology 2019, 126, 565–575. [Google Scholar] [CrossRef]
- Vezakis, I.; Lambrou, G.; Kyritsi, A.; Tagka, A.; Chatziioannou, A.; Matsopoulos, G. Detecting Skin Reactions in Epicutaneous Patch Testing withDeep Learning: An Evaluation of Pre-Processing and Modality Performance. Bioengineering 2023, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Kaliyadan, F.; Ashique, K. Use of Mobile Applications in Dermatology. Indian J. Dermatol. 2020, 65, 371. [Google Scholar] [CrossRef] [PubMed]
- Malhi, I.S.; Yiu, Z.Z.N. Algorithm-based Smartphone Apps to Assess Risk of Skin Cancer in Adults: Critical Appraisal of a Systematic Review. Br. J. Dermatol. 2021, 184, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Kyritsi, A.; Tagka, A.; Stratigos, A.; Pesli, M.; Lagiokapa, P.; Karalis, V. A Retrospective Analysis to Investigate Contact Sensitization in Greek Population Using Classic and Machine Learning Techniques. Adv. Exp. Med. Biol. 2023, 1424, 145–155. [Google Scholar] [PubMed]
- Marani, A.; Bianchelli, T.; Gesuita, R.; Faragalli, A.; Foti, C.; Malara, G.; Micali, G.; Amerio, P.; Rongioletti, F.; Corazza, M.; et al. Gender differences in adult atopic dermatitis and clinical implication: Results from a nationwide multicentre study. J. Eur. Acad. Dermatol. Venereol. 2023, 38, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.N.; Hu, K.C.; Wu, R.S.C.; Bau, D.T. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after wholebreast radiotherapy. BMC Cancer 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Pollo, C.; Miot, H.; Sousa Matos, T.; Souza, J.; Jorge, M.; Miot, L.; Meneguin, S. Prevalence and factors associated with depression and anxiety in patients with psoriasis. J. Clin. Nurs. 2021, 30, 572–580. [Google Scholar] [CrossRef]
- Uter, W.; Wilkinson, S.M.; Aerts, O.; Bauer, A.; Borrego, L.; Brans, R.; Buhl, T.; Dickel, H.; Dugonik, A.; Filon, F.L.; et al. Patch test results with the European baseline series, 2019/20-Joint European results of the ESSCA and the EBS working groups of the ESCD, and the GEIDAC. Contact Dermat. 2022, 87, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Pesonen, M.; Brans, R.; Caroppo, F.; Dickel, H.; Dugonik, A.; Filon, F.L.; Geier, J.; Gimenez-Arnau, A.M.; Napolitano, M.; et al. Occupational contact allergy: The European perspective-Analysis of patch test data from ESSCA between 2011 and 2020. Contact Dermat. 2023, 88, 263–274. [Google Scholar] [CrossRef]
- Uter, W.; Gefeller, O.; Giménez-Arnau, A.; Frosch, P.; Johansen, J.D.; Schuttelaar, M.L.; Rustemeyer, T.; Filon, F.L.; Dugonik, A.; Bircher, A.; et al. Characteristics of patients patch tested in the European Surveillance System on Contact Allergies (ESSCA) network, 2009–2012. Contact Dermat. 2015, 73, 82–90. [Google Scholar] [CrossRef]
- ESSCA Writing Group. The European Surveillance System of Contact Allergies (ESSCA): Results of patch testing the standard series, 2004. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Schnuch, A.; Wilkinson, M.; Dugonik, A.; Dugonik, B.; Gansland, T. Registries in Clinical Epidemiology: The European Surveillance System on Contact Allergies (ESSCA). Methods Inf. Med. 2016, 55, 193–199. [Google Scholar] [PubMed]
- Pesonen, M.; Jolanki, R.; Filon, F.L.; Wilkinson, M.; Kręcisz, B.; Kieć-Świerczyńska, M.; Bauer, A.; Mahler, V.; John, S.M.; Schnuch, A.; et al. Patch test results of the European baseline series among patients with occupational contact dermatitis across Europe—Analyses of the European Surveillance System on Contact Allergy network, 2002–2010. Contact Dermat. 2015, 72, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Amario-Hita, J.C.; Balato, A.; Ballmer-Weber, B.; Bauer, A.; Fortina, A.B.; Bircher, A.; Chowdhury, M.M.U.; Cooper, S.M.; Czarnecka-Operacz, M.; et al. European Surveillance System on Contact Allergies (ESSCA): Results with the European baseline series, 2013/2014. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Frosch, J.P.; Johansen, J.D.; Schuttelaar, M.L.A.; Silvestre, J.F.; Sánchez-Pérez, J.; Weisshaar, E.; Weisshaar, W.; ESSCA network. Patch test results with fragrance markers of the baseline series—Analysis of the European Surveillance System on Contact Allergies (ESSCA) network 2009–2012. Contact Dermat. 2015, 73, 163–171. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.M.; Deza, G.; Bauer, A.; Johnston, G.A.; Johnston, V.; Schuttelaar, M.L.; Sanchez-Perez, J.; Silvestre, J.F.; Wilkinson, M.; Uter, W. Contact allergy to preservatives: ESSCA* results with the baseline series, 2009–2012. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Horton, E.; Uter, W.; Geier, J.; Ballmer-Weber, B.; Bauer, A.; Bircher, A.; Dickel, H.; Giménez-Arnau, A.; Gonçalo, M.; John, S.M.; et al. Developing a cosmetic series: Results from the ESSCA network, 2009–2018. Contact Dermat. 2021, 84, 82–94. [Google Scholar] [CrossRef]
- Amann, J.; Blasimme, A.; Vayena, E.; Frey, D.; Madai, V.I. Explainability for Artificial Intelligence in Healthcare: A Multidisciplinary Perspective. BMC Med. Inform. Decis. Mak. 2020, 20, 310. [Google Scholar] [CrossRef]
- Kaul, V.; Enslin, S.; Gross, S.A. History of Artificial Intelligence in Medicine. Gastrointest. Endosc. 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Ferrara, P.; Battiato, S.; Polosa, R. Progress and Prospects for Artificial Intelligence in Clinical Practice: Learning from COVID-19. Intern. Emerg. Med. 2022, 17, 1855–1857. [Google Scholar] [CrossRef]




| Characteristic | Polysensitized Patients (N = 200, 50.0%) | Monosensitized Patients (N = 200, 50.0%) |
|---|---|---|
| Gender | ||
| Male | 100 (50.0%) | 100 (50.0%) |
| Female | 100 (50.0%) | 100 (50.0%) |
| Age (median, range) | 34.5 (18–79) | 36.7 (18–82) |
| Occupation Class | ||
| Cleaners/Householders | 23 (11.5%) | 9 (4.5%) |
| Bakers/Cooks | 13 (6.5%) | 15 (7.5%) |
| Engineers/Builders | 12 (6.0%) | 17 (8.5%) |
| Nail Technicians & Make-up Artists | 16 (8.0%) | 15 (7.5%) |
| Healthcare Workers | 19 (9.5%) | 26 (13.0%) |
| Office Workers | 79 (39.5%) | 80 (40.0%) |
| Technicians/Metal Workers | 33 (16.5%) | 31 (15.5%) |
| Hairdressers | 5 (2.5%) | 7 (3.5%) |
| MOAHLFA Index | ||
| Male (M) | 100 (50.0%) | 100 (50.0%) |
| Occupational Dermatitis (O) | 92 (46.0%) | 36 (18.0%) |
| Atopic Dermatitis (A) | 83 (41.5%) | 79 (39.5%) |
| Hand Dermatitis (H) | 128 (64.0%) | 157 (78.5%) |
| Leg Dermatitis (L) | 54 (27.0%) | 31 (15.5%) |
| Facial Dermatitis (F) | 57 (28.5%) | 79 (39.5%) |
| Age 40+ (A) | 51 (25.5%) | 65 (32.5%) |
| Trunk Dermatitis (T) | 59 (29.5%) | 37 (18.5%) |
| Atopic Dermatitis History | ||
| Family Positive History | 43 (21.5%) | 58 (29.0%) |
| Sample Size | Polysensitized Patients | Sample Size | Polysensitized Patients |
|---|---|---|---|
| (N, %) | (N = 200, 100.0%) | (N, %) | (N = 200, 100.0%) |
| Preservatives | Plastic Glues | ||
| Thimerosal 0.1% | 200 (100.0%) | Paratertiarybutyl Phenol formaldehyde | |
| Methyldibromo-Glutaronitrile 0.5% | 10 (5.0%) | Resin (BPF-Resin) 1% | 6 (3.0%) |
| KATHON 0.02% | 9 (4.5%) | 2-Hydroxyethyl-Methacrylate/HEMA 2% | 5 (2.5%) |
| Formaldehyde 2% | 8 (4.0%) | Epoxy Resin 1% | 3 (1.5%) |
| Quaternium 15 1% | 4 (2.0%) | ||
| Paraben Mix 16% | 2 (1.0%) | ||
| Medicines | Natural Origin | ||
| Ethylenediamine Dihydr 1% | 51 (25.5%) | Propolis 10% | 28 (14.0%) |
| Budesonide 0.01% | 16 (8.0%) | Sesquiterpenelactone Mix 0.1% | 6 (3.0%) |
| Neomycin Sulphate 20% | 7 (3.5%) | Colophonium 20% | 5 (2.5%) |
| Caine Mix 7% | 3 (1.5%) | Wool Alcohols 30% | 3 (1.5%) |
| Metals | Fragrances | ||
| Nickel Sulphate 5% | 95 (47.5%) | Fragrance Mix II 14% | 150 (75.0%) |
| Cobalt Chloride 1% | 26 (13.0%) | Fragrance Mix I 8% | 41 (20.5%) |
| Potassium Dichromate 0.5% | 14 (7.0%) | Balsam of Peru 25% | 23 (11.5%) |
| Dyes/Colorants | Rubbers | ||
| Paraphenylenediamine 1% | 14 (7.0%) | Thiuram Mix 1% | 11 (5.5%) |
| PPD-Black Rubber Mix 0.1% | 9 (4.5%) | Mercaptobenzothiazole (MBT) 2% | 3 (1.5%) |
| Textile Dye Mix 6.6% | 7 (3.5%) | Mercapto Mix 2% | 2 (1.0%) |
| Allergen Categories | Polysensitized Patients (N = 200, 100.0%) |
|---|---|
| Number of Allergens | |
| 3 | 109 (54.5%) |
| 4 | 49 (24.5%) |
| 5 | 27 (13.5%) |
| 6 | 7 (3.5%) |
| 7 | 4 (2.0%) |
| 8 | 2 (1.0%) |
| 9 | 1 (0.5%) |
| 11 | 1 (0.5%) |
| Most Frequent Polysensitization Patterns | |
| Preservatives/Fragrances/Metals | 110 (55.0%) |
| Preservatives/Fragrances/Medicines | 56 (28.0%) |
| Preservatives/Metals/Medicines | 28 (14.0%) |
| Preservatives/Fragrances/Metals/Medicines | 19 (9.5%) |
| Hand Dermatitis (HD) | |||||
|---|---|---|---|---|---|
| Group | Variables | Total | No | Yes | p-Value |
| All Patients | Patient Group [n (%)] | n = 400 | n = 115 | n = 285 | 0.001 |
| Polysensitized | 200 (50) | 72 (62.7) | 128 (44.9) | ||
| Monosensitized | 200 (50) | 43 (37.3) | 157 (55.1) | ||
| Polysensitized Patients | Occupation Class [n (%)] | n = 200 | n = 70 | n = 130 | 0.003 |
| Cleaners/Householders | 23 (11.5) | 6 (8.6) | 17 (13.1) | ||
| Bakers/Cooks | 13 (6.5) | 2 (2.8) | 11 (8.5) | ||
| Engineers/Builders | 12 (6.0) | 5 (7.2) | 7 (5.4) | ||
| Nail Technicians & Make-up Artists | 16 (8.0) | 6 (8.6) | 10 (7.7) | ||
| Healthcare Workers | 19 (9.5) | 4 (5.6) | 15 (11.5) | ||
| Office Workers | 79 (39.5) | 39 (55.8) | 40 (30.8) | ||
| Technicians/Metal Workers | 33 (16.5) | 7 (10.0) | 26 (20.0) | ||
| Hairdressers | 5 (2.5) | 1 (1.4) | 4 (3.0) | ||
| Polysensitized Patients | Gender [n (%)] | n = 200 | n = 72 | n = 128 | 0.077 |
| Males | 100 (50) | 30 (41.7) | 70 (54.7) | ||
| Females | 100 (50) | 42 (58.3) | 58 (45.3) | ||
| Monosensitized Patients | Occupation Class [n (%)] | n = 200 | n = 43 | n = 157 | 0.000 |
| Cleaners/Householders | 9 (4.5) | 1 (2.3) | 8 (5.0) | ||
| Bakers/Cooks | 15 (7.5) | 0 (0) | 15 (9.6) | ||
| Engineers/Builders | 17 (8.5) | 1 (2.3) | 16 (10.1) | ||
| Nail Technicians and Make-up Artists | 15 (7.5) | 1 (2.3) | 14 (9.0) | ||
| Healthcare Workers | 26 (13.0) | 2 (4.6) | 24 (15.2) | ||
| Office Workers | 80 (40.0) | 32 (74.5) | 48 (30.6) | ||
| Technicians/Metal Workers | 31 (15.5) | 5 (11.7) | 26 (16.6) | ||
| Hairdressers | 7 (3.5) | 1 (2.3) | 6 (3.9) | ||
| Monosensitized Patients | Gender [n (%)] | n = 200 | n = 43 | n = 157 | 0.025 |
| Males | 100 (50) | 15 (34.9) | 85 (54.1) | ||
| Females | 100 (50) | 28 (65.1) | 72 (45.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyritsi, A.; Tagka, A.; Stratigos, A.; Karalis, V.D. Machine Learning in Allergic Contact Dermatitis: Identifying (Dis)similarities between Polysensitized and Monosensitized Patients. BioMedInformatics 2024, 4, 1348-1362. https://doi.org/10.3390/biomedinformatics4020074
Kyritsi A, Tagka A, Stratigos A, Karalis VD. Machine Learning in Allergic Contact Dermatitis: Identifying (Dis)similarities between Polysensitized and Monosensitized Patients. BioMedInformatics. 2024; 4(2):1348-1362. https://doi.org/10.3390/biomedinformatics4020074
Chicago/Turabian StyleKyritsi, Aikaterini, Anna Tagka, Alexander Stratigos, and Vangelis D. Karalis. 2024. "Machine Learning in Allergic Contact Dermatitis: Identifying (Dis)similarities between Polysensitized and Monosensitized Patients" BioMedInformatics 4, no. 2: 1348-1362. https://doi.org/10.3390/biomedinformatics4020074







