MOF-Derived ZrO2-Supported Bimetallic Pd–Ni Catalyst for Selective Hydrogenation of 1,3-Butadiene
Abstract
:1. Introduction
2. Results and Discussion
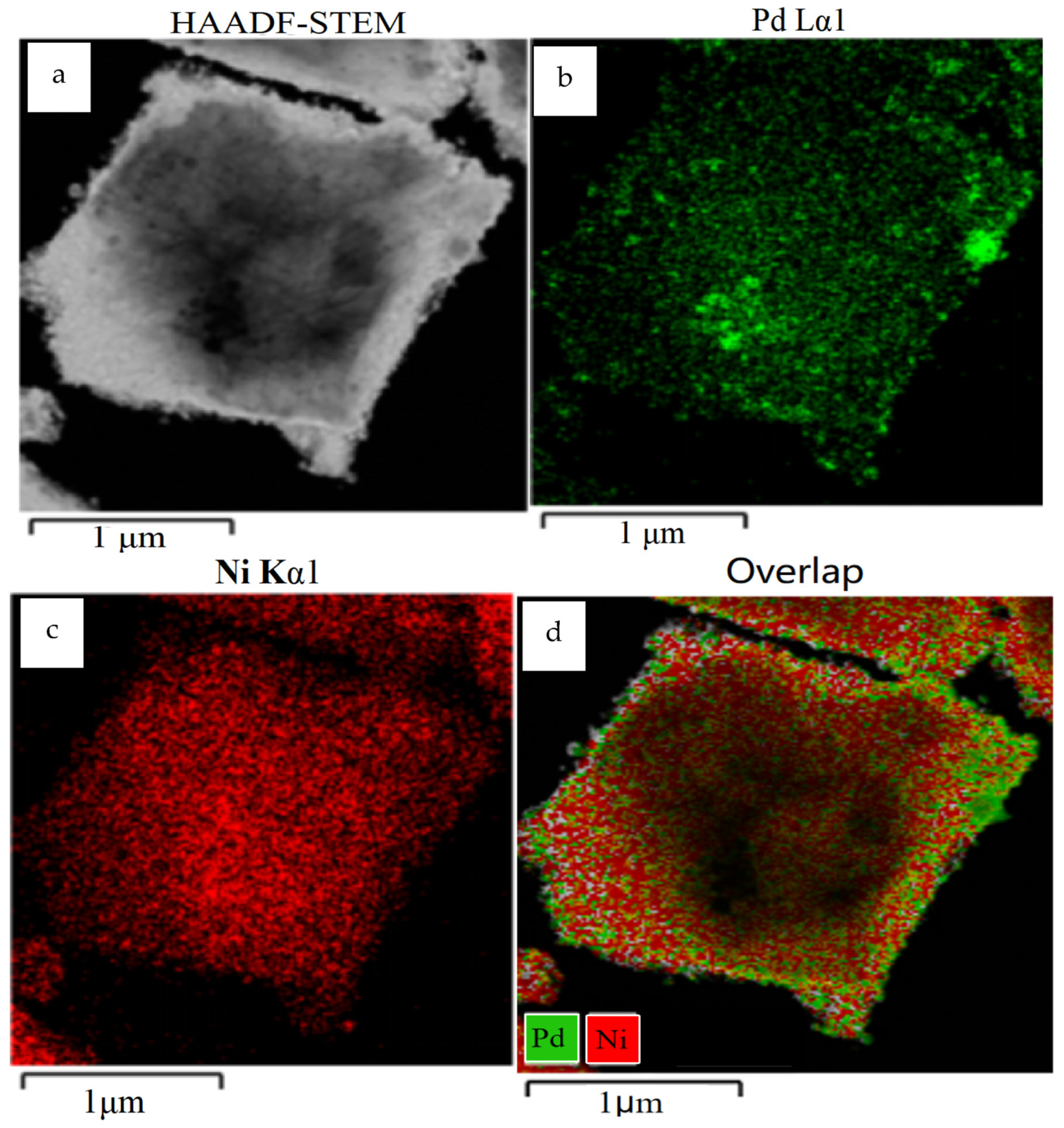
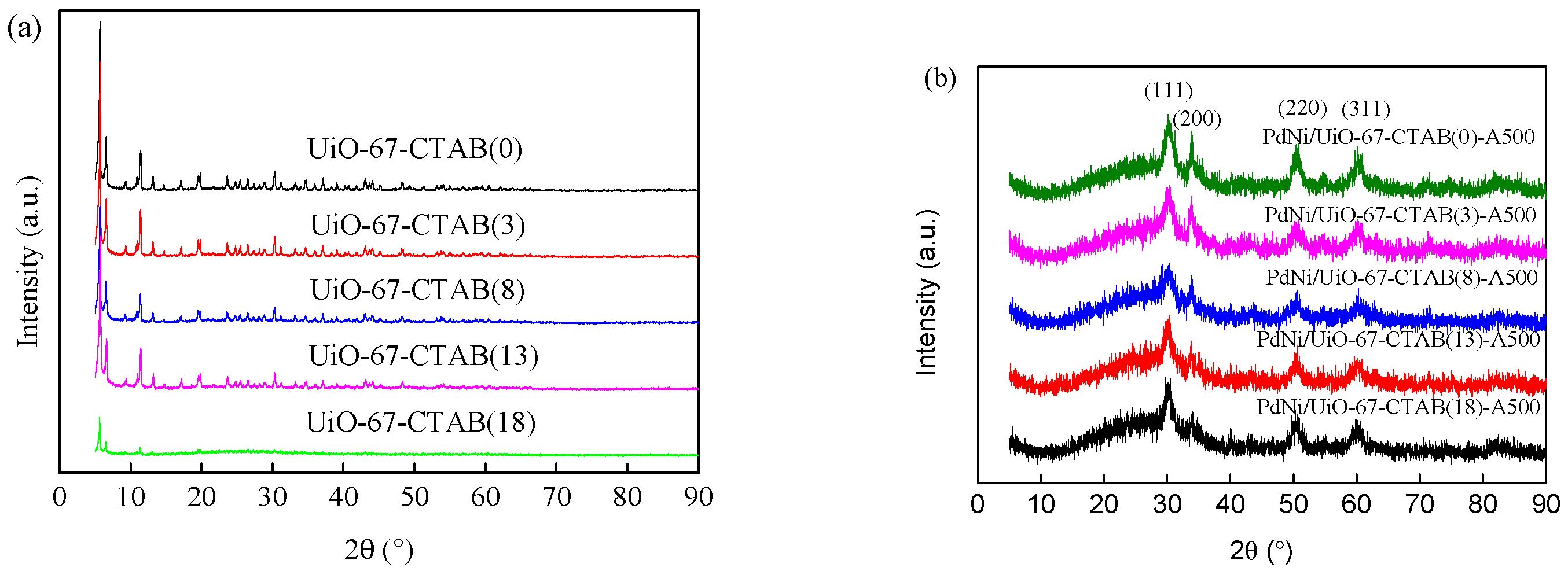
2.1. Synthesis and Characterization
2.2. Catalytic Performance and Stability
3. Experimental Section
3.1. Synthesis of UiO-67-CTAB
3.2. Synthesis of PdNi/UiO-67-CTAB-A500
3.3. Catalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Sun, K.; Xu, Y.; Ding, Z.; Hou, R. Tuning crystal phase of molybdenum carbide catalyst to induce the different selective hydrogenation performance. Appl. Catal. A Gen. 2022, 630, 118455–118465. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, R.; Sun, K. Tuning butene selectivities by Cu modification on Pd-based catalyst for the selective hydrogenation of 1,3-butadiene. J. Catal. 2019, 374, 12–23. [Google Scholar] [CrossRef]
- Hugon, A.; Delannoy, L.; Krafft, J.M.; Louis, C. Selective hydrogenation of 1,3-butadiene in the presence of an excess of alkenes over supported bimetallic gold-palladium catalysts. J. Phys. Chem. C 2010, 114, 10823–10835. [Google Scholar] [CrossRef]
- Hu, N.; Li, X.Y.; Liu, S.M.; Wang, Z.; He, X.K.; Hou, Y.X.; Wang, Y.X.; Deng, Z.; Chen, L.H.; Su, B.L. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation. Chin. J. Catal. 2020, 41, 1081–1090. [Google Scholar] [CrossRef]
- Hu, C.; Shao, M.; Xiang, M.; Li, S.; Xu, S. The role of hydrogen coverage and location in 1,3-butadiene hydrogenation over Pt/SiO2. React. Chem. Eng. 2020, 5, 87–100. [Google Scholar] [CrossRef]
- Yan, H.; Cheng, H.; Yi, H.; Lin, Y.; Yao, T.; Wang, C.; Li, J.; Wei, S.; Lu, J. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 2015, 137, 10484–10487. [Google Scholar] [CrossRef] [PubMed]
- Corstius, O.E.B.; van der Hoeven, J.E.S.; Sunley, G.J.; de Jongh, P.E. Influence of particle size in Pd-catalysed selective hydrogenation of 1,3-butadiene. J. Catal. 2023, 427, 115103–115111. [Google Scholar] [CrossRef]
- Lu, F.; Xu, Y.; Jiang, X.; Liu, Y.; Huang, J.; Sun, D. Biosynthesized Pd/gamma-Al2O3 catalysts for low-temperature 1,3-butadiene hydrogenation: The effect of calcination atmosphere. New J. Chem. 2017, 41, 13036–13042. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, F.; Wu, J.; Li, Q.; Sun, D. Bio-inspired n self-doped 3d macroporous carbon supported Pd nanoparticles as an efficient catalyst for selective hydrogenation of 1,3-butadiene. J. Taiwan Inst. Chem. Eng. 2022, 136, 104391–104396. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Hoang, L.; Kumar, V.V.; Dumbre, D.; Bhargava, S.K. Selective hydrogenation of 1,3-butadiene to 1-butene: Review on catalysts, selectivity, kinetics and reaction mechanism. Catal. Clean Energy Environ. Sustain. 2021, 2, 205–228. [Google Scholar]
- Wang, M.; Wang, Y.; Mou, X.; Lin, R.; Ding, Y. Design strategies and structure-performance relationships of heterogeneous catalysts for selective hydrogenation of 1,3-butadiene. Chin. J. Catal. 2022, 43, 1017–1041. [Google Scholar] [CrossRef]
- Yan, Z.; Cui, L.; Pang, Z.; Shi, K.; Zhang, M.; Guo, J.; Gao, R.; Hao, H. Theoretical study the catalytic performance and mechanism of novel designed single atom catalysts M1/2DMs for 1,3-butadiene hydrogenation. Appl. Surf. Sci. 2023, 617, 156585–156596. [Google Scholar] [CrossRef]
- Huang, J.; Odoom-Wubah, T.; Jing, X.; Sun, D.; Gu, Z.; Li, Q. Plant-mediated synthesis of zinc oxide supported nickel palladium alloy catalyst for the selective hydrogenation of 1,3-butadiene. ChemCatChem 2017, 9, 870–881. [Google Scholar] [CrossRef]
- Yan, Z.; Cui, L.; Shi, K.; Zhang, M.; Pang, Z.; Guo, J.; Gao, R.; Hao, H. Theoretical study the influence of partial substitute noble metal Pd/Ag of PdAg-based catalyst by non-noble metal Ni/Cu for 1,3-Butadiene hydrogenation. Appl. Surf. Sci. 2022, 588, 152897–152903. [Google Scholar] [CrossRef]
- Heemeier, M.; Carlsson, A.F.; Naschitzki, M.; Schmal, M.; Baumer, M.; Freand, H.J. Preparation and characterization of a model bimetallic catalyst: Co–Pd nanoparticles supported on Al2O3. Angew. Chem. Int. Ed. 2002, 41, 4073–4076. [Google Scholar] [CrossRef]
- Méndez, F.J.; Piccolo, L.; Solano, R.; Aouine, M.; Villasana, Y.; Guerra, J.; Curbelo, S.; Olivera-Fuentes, C.; Brito, J.L. Promoting effect of ceria on the performance of NiPd/CeO2-Al2O3 catalysts for the selective hydrogenation of 1,3-butadiene in the presence of 1-butene. New J. Chem. 2018, 42, 11165–11173. [Google Scholar] [CrossRef]
- Ma, H.; Xu, X.; Xu, H.; Feng, H.; Xie, Y.; Cheng, D. Understanding composition-dependent catalytic performance of PdAg for the hydrogenation of 1,3-butadiene to 1-butene. Catal. Commun. 2021, 149, 106255–106260. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Jiang, X. Plant-mediated synthesis of AgPd/γ-Al2O3 catalysts for selective hydrogenation of 1,3-butadiene at low temperature. New J. Chem. 2019, 43, 13891–13898. [Google Scholar] [CrossRef]
- Lv, C.Q.; Liu, J.H.; Guo, Y.; Wang, G.C. Selective hydrogenation of 1,3-butadiene over single Pt1/Cu(1 1 1) model catalysts: A DFT study. Appl. Surf. Sci. 2019, 466, 946–955. [Google Scholar] [CrossRef]
- Liu, L.; Yu, L.; Zhou, X.; Xin, C.; Sun, S.; Liu, Z.; Zhang, J.; Liu, Y.; Tai, X. Comparative study of Pd–Ni bimetallic catalysts supported on UiO-66 and UiO-66-NH2 in selective 1,3-butadiene hydrogenation. Nanomaterials 2022, 12, 1484. [Google Scholar] [CrossRef]
- Hou, R.; Porosoff, M.D.; Chen, J.G.; Wang, T. Effect of oxide supports on Pd-Ni bimetallic catalysts for 1,3-butadiene hydrogenation. Appl. Catal. A-Gen. 2015, 490, 17–23. [Google Scholar] [CrossRef]
- Sarkany, A.; Zsoldos, Z.; Stefler, G.; Hightower, J.W.; Guczi, L. Promoter effect of Pd in hydrogenation of 1,3-butadiene over Co-Pd catalysts. J. Catal. 1995, 157, 179–189. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Guo, L.; Yan, S.; Li, Y.; Jiang, S.; Tai, X. Bimetallic Au–Pd alloy nanoparticles supported on MIL-101(Cr) as highly efficient catalysts for selective hydrogenation of 1,3-butadiene. RSC Adv. 2020, 10, 33417–33427. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Dai, J.; Liu, X.; Tan, K.B.; Huang, J.; Zhan, G.; Li, Q.; Acquisition, F. Waste eggshell with naturally-functionalized sulfonic groups as excellent support for loading Pd and Ag nanoparticles towards enhanced 1,3-butadiene hydrogenation. Mol. Catal. 2021, 510, 111689–111698. [Google Scholar] [CrossRef]
- Feng, X.; Wu, D.; Shen, X.; Guo, Y.; Lv, Y.; Xu, A.; Li, X. Activation of sulfite by metal-organic framework-derived cobalt nanoparticles for organic pollutants removal. J. Environ. Sci. 2023, 124, 350–359. [Google Scholar] [CrossRef]
- Han, Y.C.; Liu, M.L.; Sun, L.; Li, X.C.; Yao, Y.; Zhang, C.; Ding, S.Y.; Liao, H.G.; Zhang, L.; Fan, F.R.; et al. A general strategy for overcoming the trade-off between ultrasmall size and high loading of MOF-derived metal nanoparticles by millisecond pyrolysis. Nano Energy 2022, 97, 107125–107134. [Google Scholar] [CrossRef]
- Torkashvand, Z.; Sepehrmansourie, H.; Zolfigol, M.A.; As’Habi, M.A. Application of Ti-MOF-UR as a new porous catalyst for the preparation of pyrazolo [3,4-b]quinoline and pyrazolo [4,3-e]pyridines. Mol. Catal. 2023, 541, 113107–113118. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, A.; Liu, P.; Zhang, Z.; Yu, F.; Qi, W.; Li, B. Application of copper(II)-organic frameworks bearing dilophine derivatives in photocatalysis and guest separation. Inorg. Chem. 2022, 61, 16009–16019. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ru, R.; Mao, J.; Meng, Q.; Fan, Z.; Li, X.; Zhang, G. Assembly of MOFs/polymer hydrogel derived Fe3O4-CuO@hollow carbon spheres for photochemical oxidation: Freezing replacement for structural adjustment. Appl. Catal. B-Environ. 2020, 269, 118754–118763. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, Y.; Wang, S.; Hu, Z.; Liu, Q.; Zheng, X. Construction of highly dispersed Pt single sites and high-efficiency heterocatalysis silylation of alcohols with silanes. Nano Res. 2023, 16, 4643–4649. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, H.; Hai, G.; Zeng, Y.; Wang, X.; Xing, L.; Zhao, J.; Wang, G.; Shu, X. Base-free catalytic aerobic oxidation of mercaptans over MOF-derived Co/CN catalyst with controllable composition and structure. J. Colloid Interface Sci. 2022, 607, 1836–1848. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Q.Y.; Wang, X.J.; Zheng, J.; Li, X.G. MOF-derived surface modified Ni nanoparticles as an efficient catalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 16435–16439. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.-J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Zeng, D.; Li, Y.; Ma, H.; Cui, F.; Zhang, J. CuO@NiO nanoparticles derived from metal-organic framework precursors for the deoxygenation of fatty acids. ACS Sustain. Chem. Eng. 2021, 9, 15612–15622. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Liang, Q.; Zhou, M.; Li, Z.; Xu, S. Coupling MOF-derived titanium oxide with CdIn2S4 formed 2D/3D core–shell heterojunctions with enhanced photocatalytic performance. Sep. Purif. Technol. 2021, 279, 119765–119779. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, M.; Tong, J.; Peng, H.; Xiang, Y.; Chen, Z.; Xu, Z.; Yang, Z.; Xiong, W. Coupling effects between metal–organic framework derivatives and oxygen-deficient TiO2 nanotubes: Identified charge-transfer processes and photoelectric synergistic effect. Environ. Sci. Nano 2023, 10, 1993–2009. [Google Scholar] [CrossRef]
- Wang, H.; Sun, C.; Zhu, E.; Shi, C.; Yu, J.; Xu, M. Core-shell MOF-derived Fe3C-Co-NC as high-performance ORR/OER bifunctional catalyst. J. Alloys Compd. 2023, 948, 169728–169737. [Google Scholar] [CrossRef]
- Boroujerdian, M.; Rahimi, S.; Nezhad, S.M.; Pourmousavi, S.A.; Zare, E.N.; Salimi, F.; Amirahmadi, F.; Daneshgar, H. CoFe2O4@SiO2–NH2@MOF-5 magnetic nanocatalyst for the synthesis of biologically active quinazoline derivatives. Environ. Res. 2023, 236, 116708–116722. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wang, S.; Li, J.; Xu, Y.; Li, S. Different effect of Y (Y = Cu, Mn, Fe, Ni) doping on Co3O4 derived from Co-MOF for toluene catalytic destruction. Chem. Eng. Sci. 2022, 251, 117436–117444. [Google Scholar] [CrossRef]
- Lei, J.; Wang, P.; Wang, S.; Li, J.; Xu, Y.; Li, S. Enhancement effect of Mn doping on Co3O4 derived from Co-MOF for toluene catalytic oxidation. Chin. J. Chem. Eng. 2022, 52, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Bo, K.; Wang, Y.; Wang, Y. Eutectic salts assisted synthesis of MOF-derived porous carbon as Pt-Sn catalyst support for ethanol oxidation reaction. Int. J. Hydrogen Energy 2022, 47, 18285–18293. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, F.; Wang, Y.; Jia, M.; Tao, X.; Jin, Y.; Zhang, X. MOF-derived CeO2 supported Ag catalysts for toluene oxidation: The effect of synthesis method. Mol. Catal. 2021, 515, 111922–111932. [Google Scholar] [CrossRef]
- Beiranvand, M.; Habibi, D.; Khodakarami, H. Novel UiO-NH2-like Zr-based MOF (Basu-DPU) as an excellent catalyst for preparation of new 6H-chromeno [4,3-b]quinolin-6-ones. ACS Omega 2023, 8, 25924–25937. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, Z. Determination of water-soluble nitrate ions in PM2.5 particles using UiO-67 modified glassy carbon electrode. Int. J. Electrochem. Sci. 2022, 17, 2–12. [Google Scholar] [CrossRef]
- Liu, L.; Tai, X.; Zhou, X.; Liu, L.; Zhang, X.; Ding, L.; Zhang, Y. Au-Pt bimetallic nanoparticle catalysts supported on UiO-67 for selective 1,3-butadiene hydrogenation. J. Taiwan Inst. Chem. Eng. 2020, 114, 220–227. [Google Scholar] [CrossRef]
- Samperisi, L.; Jaworski, A.; Kaur, G.; Lillerud, K.P.; Zou, X.; Huang, Z. Probing molecular motions in metal-organic frameworks by three dimensional electron diffraction. J. Am. Chem. Soc. 2021, 143, 17947–17952. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dong, M.; Gou, W.; Li, J.; Qin, Z.; Wang, J.; Fan, W. Rapid tuning of ZSM-5 crystal size by using polyethylene glycol or colloidal silicalite-1 seed. Microporous Mesoporous Mater. 2012, 163, 192–200. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Liu, Q.; Ren, Y.; Jiang, H. Visible light-driven efficient palladium catalyst turnover in oxidative transformations within confined frameworks. Nat. Commun. 2022, 13, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cairnie, D.R.; Chakraborty, A.; Cai, M.; Morris, A.J. Photoelectrochemical alcohol oxidation by mixed-linker metal organic frameworks. Faraday Discuss. 2021, 225, 371–383. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yang, J.J.; Yan, Y.L. Novel adsorption-photocatalysis integrated bismuth tungstate modified layered mesoporous titanium dioxide (Bi2WO6/LM-TiO2) composites. Opt. Mater. 2022, 130, 112581–112590. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Sun, C.; Zhao, T.; Zhao, J.; Wang, Z.; Liu, W.; Wu, S.; Shi, M.; Bu, L. Novel synthesis and catalytic performance of hierarchical MOR. New J. Chem. 2021, 45, 8629–8638. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Sheng, Y.; Zhu, H.; Li, Z.; Su, Z.; Yu, R.; Zhang, S. Synthesis of a crystalline zeolitic imidazole framework-8 nano-coating on single environment-sensitive viral particles for enhanced immune responsed. Nanoscale Adv. 2023, 5, 1433–1449. [Google Scholar] [CrossRef]
- Sadeh, P.; Zeinali, S.; Rastegari, B.; Najafipour, I. Size optimization of mesoporous β-cyclodextrin metal-organic frameworks as bio-MOFs. J. Cryst. Growth 2023, 620, 127348–127354. [Google Scholar] [CrossRef]
- Ahmadi, H.; Khalaj, G.; Soleymani, F.; Moalem, M.; Pourabdollah, M.; Mahmoudan, M. Electrochemical synthesis and characterization of Cu2O nanoparticles: Effect of electrolyte composition. J. Solid State Electrochem. 2023, 1–13. [Google Scholar] [CrossRef]
- He, P.; Li, Y.; Cai, K.; Xiong, X.; Lv, J.; Wang, Y.; Huang, S.; Ma, X. Nano-assembled mordenite zeolite with tunable morphology for carbonylation of dimethyl ether. ACS Appl. Nano Mater. 2020, 3, 6460–6468. [Google Scholar] [CrossRef]
- Jubeer, E.M.; Manthrammel, M.A.; Shkir, M.; Subha, P.A.; Yahia, I.S.; Alfaify, S.A. Microwave assisted synthesis of quantum dots like ZnS nanoparticles for optoelectronic applications: An effect of CTAB concentrations. Optik 2021, 240, 166812–166820. [Google Scholar] [CrossRef]
- Ping, C.; Chen, X.; Qin, F.; Ma, W. Effect of preparation conditions on the morphology and catalytic performance of nickel phosphate nanotubes. Cryst. Res. Technol. 2022, 57, 2100273–2100281. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Xin, C.; Zhang, B.; Zhang, G.; Li, S.; Liu, L.; Tai, X. Efficient oxidation of benzyl alcohol into benzaldehyde catalyzed by graphene oxide and reduced graphene oxide supported bimetallic Au–Sn catalysts. RSC Adv. 2023, 13, 23648–23658. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, Z.; Lv, Y.; Xin, C.; Zhou, X.; Yu, L.; Tai, X. MIL-100(Fe) supported PtCo nanoparticles as active and selective heterogeneous catalysts for hydrogenation of 1,3-butadiene. ChemistryOpen 2022, 11, e202100288. [Google Scholar] [CrossRef]
- Ren, H.; Cheng, L.; Yang, J.; Zhao, K.; Zhai, Q.; Li, Y. Recyclable and reusable chiral α, α-L-diaryl prolinol heterogeneous catalyst grafting to UiO-67 for enantioselective hydration/aldol/oxa-Diels Alder domino reaction. Catal. Commun. 2021, 149, 106249–106254. [Google Scholar] [CrossRef]
- Melillo, A.; García-Aboal, R.; Navalón, S.; Atienzar, P.; Ferrer, B.; Álvaro, M.; García, H. Photoactive Zr and Ti metal-organic-frameworks for solid-state solar cells. ChemPhysChem 2021, 22, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, M.; Chang, X.; Hao, Z.; Chen, J.; Pan, Y.; Kawi, S.; Ma, X. Hollow structured Cu@ZrO2 derived from Zr-MOF for selective hydrogenation of CO2 to methanol. J. Energy Chem. 2022, 71, 277–287. [Google Scholar] [CrossRef]
- Palani, R.; Wu, Y.S.; Wu, S.H.; Jose, R.; Yang, C.C. Metal-organic framework-derived ZrO2/NiCo2O4/graphene mesoporous cake-like structure as enhanced bifunctional electrocatalytic cathodes for long life Li-O2 batteries. Electrochim. Acta 2022, 412, 140147–140159. [Google Scholar] [CrossRef]
- Alqarni, D.S.; Marshall, M.; Gengenbach, T.R.; Lippi, R.; Chaffee, A.L. Rh/ZrO2@C(MIL) catalytic activity and TEM images. CO2 conversion performance and structural systematic evaluation of novel catalysts derived from Zr-MOF metallated with Ru, Rh, Pd or In. Microporous Mesoporous Mater. 2022, 336, 111855–111960. [Google Scholar] [CrossRef]
- Numpilai, T.; Kidkhunthod, P.; Cheng, C.K.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. CO2 hydrogenation to methanol at high reaction temperatures over In2O3/ZrO2 catalysts: Influence of calcination temperatures of ZrO2 support. Catal. Today 2021, 375, 298–306. [Google Scholar] [CrossRef]
- Martínez-Martínez, R.; Juárez-López, G.; García-Hipólito, M.; Díaz, J.B.; Téllez, S.C.; Aguilar-Frutis, M.A.; Flores, G.A.; Falcony, C. Blue and bluish-white colors from the luminescent ZrO2 and ZrO2: Al3+ films prepared by the USP method. Mater. Res. Express 2021, 8, 016201–016214. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Liu, L.; Jiang, S.; Li, Y.; Guo, L.; Yan, S.; Tai, X. Heterogeneous bimetallic Cu-Ni nanoparticle-supported catalysts in the selective oxidation of benzyl alcohol to benzaldehyde. Catalysts 2019, 9, 538. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, X.; Huang, S.; Wu, H.; Jiang, L.; Zhou, Y.; Song, Y.; Diao, Y. Effects of MgO doping in Pd/γ-Al2O3 catalysts for the hydrogenation of perfluoro olefin. Mol. Catal. 2024, 552, 113652–113661. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, X.; Liang, B.; Fan, X.; Wei, X.; Liang, J.; Wang, L. Embedding MIL-100(Fe) with magnetically recyclable Fe3O4 nanoparticles for highly efficient esterification of diterpene resin acids and the associated kinetics. Microporous Mesoporous Mater. 2019, 289, 109615–109624. [Google Scholar] [CrossRef]
- Liu, L.; Tai, X.; Zhou, X.; Hou, J.; Zhang, Z. Bimetallic Au-Ni alloy nanoparticles in a metal-organic framework (MIL-101) as effificient heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde. J. Alloys Compd. 2019, 790, 326–336. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Zhou, Z.; Chen, K.; Wu, M.; Yuan, L. High dispersed Pd supported on CeO2 (1 0 0) for CO oxidation at low temperature. Mol. Catal. 2021, 508, 111580–111587. [Google Scholar] [CrossRef]
- Osuga, R.; Takeuchi, T.; Sawada, M.; Kunitake, Y.; Matsumoto, T.; Yasuda, S.; Onozuka, H.; Tsutsuminai, S.; Kondo, J.N.; Gies, H.; et al. Fabrication of AEI-type aluminosilicate catalyst with sheet-like morphology for direct conversion of propene to butenes. Catal. Sci. Technol. 2021, 11, 5839–5848. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Zhang, L.; Han, J.; Wang, G.; Fan, F.; Wang, T.; Zhang, X.; Fu, Y. Size and morphology control of two-dimensional metal-organic frameworks through coordination modulation. Microporous Mesoporous Mater. 2023, 348, 112379–112385. [Google Scholar] [CrossRef]
- Wu, M.; Miao, M.; Li, W.; Zhang, X.; Zhang, L.; Zhen, T.; Fu, Y.; Jin, J.; Yuan, L. Metal-organic framework-derived one-dimensional Pd@CeO2 catalysts with enhanced activity for methane oxidation. Fuel 2023, 331, 125575–125584. [Google Scholar] [CrossRef]
- Zou, H.H.; Yuan, C.Z.; Zou, H.Y.; Cheang, T.Y.; Zhao, S.J.; Qazi, U.Y.; Zhong, S.L.; Wang, L.; Xu, A.W. Bimetallic phosphide hollow nanocubes derived from a prussian-blue-analog used as highperformance catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2017, 7, 1549–1555. [Google Scholar] [CrossRef]
- Lu, W.; Wang, J.; Ma, Z.; Chen, C.; Liu, Y.; Hou, B.; Li, D.; Wang, B. Classifying and understanding the role of carbon deposits on cobalt catalyst for Fischer-Tropsch synthesis. Fuel 2023, 332, 126115–126121. [Google Scholar] [CrossRef]
- Duan, J.; He, Z.; Ma, Z.; Yue, J.; Wang, W.; Li, C. The composite of Zr-doped TiO2 and MOF-derived metal oxide for oxidative removal of formaldehyde at the room temperature. Microporous Mesoporous Mater. 2022, 336, 111892–111901. [Google Scholar] [CrossRef]
- Mo, W.L.; Ren, Y.; Ma, Y.; Guo, J.; Feng, Z.H.; Zhang, S.P.; Yang, X.Q. Structure characteristics and removal behavior of the deposited carbon on Ni-Al2O3 catalyst for CO2 reforming of CH4. Processes 2023, 11, 2968. [Google Scholar] [CrossRef]
- Chai, J.; Pestman, R.; Chiang, F.K.; Men, Z.; Wang, P.; Hensen, E.J.M. Influence of carbon deposits on Fe-carbide for the Fischer-Tropsch reaction. J. Catal. 2022, 416, 289–300. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, D.; Yu, J.; Jiang, X.; Huang, J.; Sun, D. Plant-mediated synthesis of Pd catalysts toward selective hydrogenation of 1,3-butadiene: The effect of halide ions. Ind. Eng. Chem. Res. 2017, 56, 10623–10630. [Google Scholar] [CrossRef]
- Pattamakomsan, K.; Ehret, E.; Morfin, F.; Gelin, P.; Jugnet, Y.; Prakash, S.; Bertolini, J.C.; Panpranot, J.; Aires, F.J.C.S. Selective hydrogenation of 1,3-butadiene over Pd and Pd-Sn catalysts supported on different phases of alumina. Catal. Today 2011, 164, 28–33. [Google Scholar] [CrossRef]
- Asedegbega-Nieto, E.; Iglesias-Juez, A.; Di Michiel, M.; Fernandez-Garcia, M.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Dynamics of Pd subsurface hydride formation and their impact on the selectivity control for selective butadiene hydrogenation reaction. Nanomaterials 2023, 13, 1099. [Google Scholar] [CrossRef]
- van der Hoeven, J.E.S.; Jelic, J.; Olthof, L.A.; Totarella, G.; van Dijk-Moes, R.J.A.; Krafft, J.M.; Louis, C.; Studt, F.; van Blaaderen, A.; de Jongh, P.E. Unlocking synergy in bimetallic catalysts by core–shell design. Nat. Mater. 2021, 20, 1216–1220. [Google Scholar] [CrossRef]
- Odoom-Wubah, T.; Li, Q.; Chen, M.; Fang, H.; Asare Bediako, B.B.; Adilov, I.; Huang, J.; Li, Q. Influence of preparation methods on the catalytic activity of Pd–Cu/Mn2O3 catalyst in the hydrogenation of 1,3-butadiene. ACS Omega 2019, 4, 1300–1310. [Google Scholar] [CrossRef]
- Yi, H.; Du, H.; Hu, Y.; Yan, H.; Jiang, H.L.; Lu, J. Precisely controlled porous alumina overcoating on Pd catalyst by atomic layer deposition: Enhanced selectivity and durability in hydrogenation of 1,3-butadiene. ACS Catal. 2015, 5, 2735–2739. [Google Scholar] [CrossRef]
- Sárkány, A. Features of deposit formation from 1,3-butadiene over Pd catalysts. React. Kinet. Catal. Lett. 1999, 68, 153–163. [Google Scholar] [CrossRef]
- Frusteri, F.; Spadaro, L.; Arena, F.; Chuvilin, A. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni/MgO catalysts during the dry reforming of methane. Carbon 2002, 40, 1063–1070. [Google Scholar] [CrossRef]
- Liu, F.; Li, H.; Fu, Q.; He, X.; Zhang, W. ZrSi2-SiC/SiC gradient coating of micro-structure and anti-oxidation property on C/C composites prepared by SAPS. Coatings 2022, 12, 1377. [Google Scholar] [CrossRef]










| Entry | Sample | SBET (m2/g) | Vtotal (cm3/g) |
|---|---|---|---|
| 1 | UiO-67-CTAB(0) | 686.1 | 0.40 |
| 2 | UiO-67-CTAB(3) | 264.9 | 0.17 |
| 3 | UiO-67-CTAB(8) | 1402.2 | 0.73 |
| 4 | UiO-67-CTAB(13) | 1611.8 | 0.83 |
| 5 | UiO-67-CTAB(18) | 1309.1 | 0.70 |
| 6 | PdNi/UiO-67-CTAB(0)-A500 | 60.9 | 0.09 |
| 7 | PdNi/UiO-67-CTAB(3)-A500 | 54.6 | 0.09 |
| 8 | PdNi/UiO-67-CTAB(8)-A500 | 66.7 | 0.11 |
| 9 | PdNi/UiO-67-CTAB(13)-A500 | 62.4 | 0.09 |
| 10 | PdNi/UiO-67-CTAB(18)-A500 | 54.3 | 0.09 |
| Entry | Catalyst | Pd Loading (wt%) | Pd Size (nm) | T (°C) | Conv. (%) | Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | PdNi/UiO-67-CTAB(3)-A500 | 6.5 | 6.7 | 40 | 98.4 | 44.8 | this work |
| 2 | Pd/pollen-C-500 | 1 | 112.6 | 50 | 23.0 | 97.9 | 9 |
| 3 | 1PdGONE | 1 | 8.9 | 50 | 15 | 88 | 82 |
| 4 | 2PdG | 2 | 4.9 | 50 | 89 | 27 | 82 |
| 5 | Ni1-Pd1/ZnO | - | 3.6 | 35 | 98.8 | 90.0 | 13 |
| 6 | Ni1-Pd1/SiO2 | - | 3.2 | 35 | 44.0 | 93.8 | 13 |
| 7 | Pd-Ag/ES | 0.5 | 3.7 | 45 | 95.0 | 95.8 | 24 |
| 8 | Au@Pd@SiO2 | 0.02 | monodisperse | 35 | 98.0 | 80.0 | 83 |
| 9 | Pd–Cu/Mn2O3 | 1 | 3.8 | 50 | 99.1 | 92.0 | 84 |
| 10 | PdNi/UiO-66 (1:1) | 2.65 | 7.1 | 40 | 99.8 | 84.5 | 20 |
| 11 | PdNi/UiO-66-NH2 (1:1) | 2.51 | 4.6 | 40 | 15.6 | 99.5 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, L.; Wang, L.; Zang, M.; Li, L.; Zhang, Y. MOF-Derived ZrO2-Supported Bimetallic Pd–Ni Catalyst for Selective Hydrogenation of 1,3-Butadiene. Molecules 2024, 29, 2217. https://doi.org/10.3390/molecules29102217
Liu Y, Liu L, Wang L, Zang M, Li L, Zhang Y. MOF-Derived ZrO2-Supported Bimetallic Pd–Ni Catalyst for Selective Hydrogenation of 1,3-Butadiene. Molecules. 2024; 29(10):2217. https://doi.org/10.3390/molecules29102217
Chicago/Turabian StyleLiu, Ying, Lili Liu, Leyuan Wang, Miaoliang Zang, Lei Li, and Yunkai Zhang. 2024. "MOF-Derived ZrO2-Supported Bimetallic Pd–Ni Catalyst for Selective Hydrogenation of 1,3-Butadiene" Molecules 29, no. 10: 2217. https://doi.org/10.3390/molecules29102217





