Enhancing Cr (VI) Adsorption of Chestnut Shell Biochar through H3PO4 Activation and Nickel Doping
Abstract
:1. Introduction
2. Results and Discussion
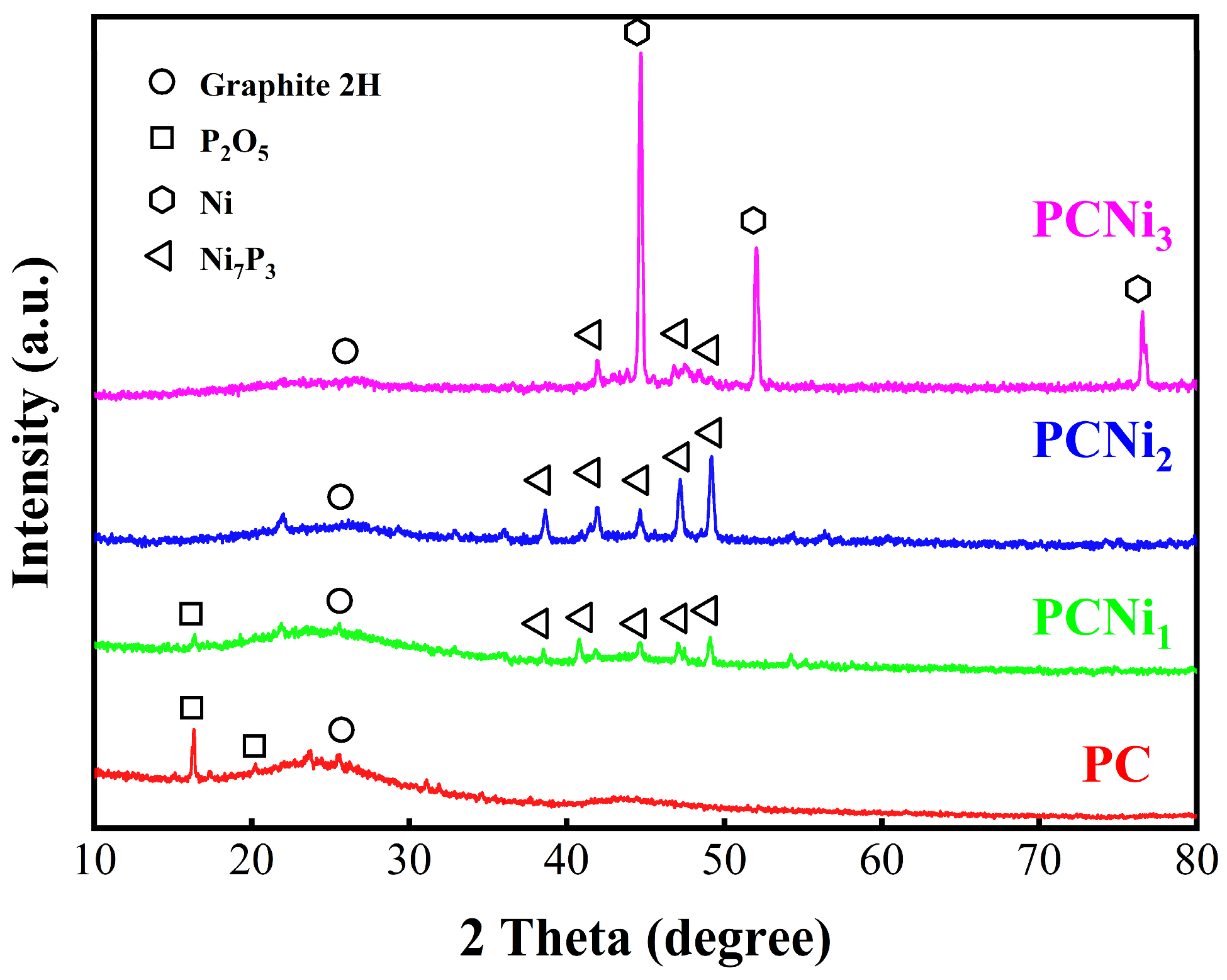
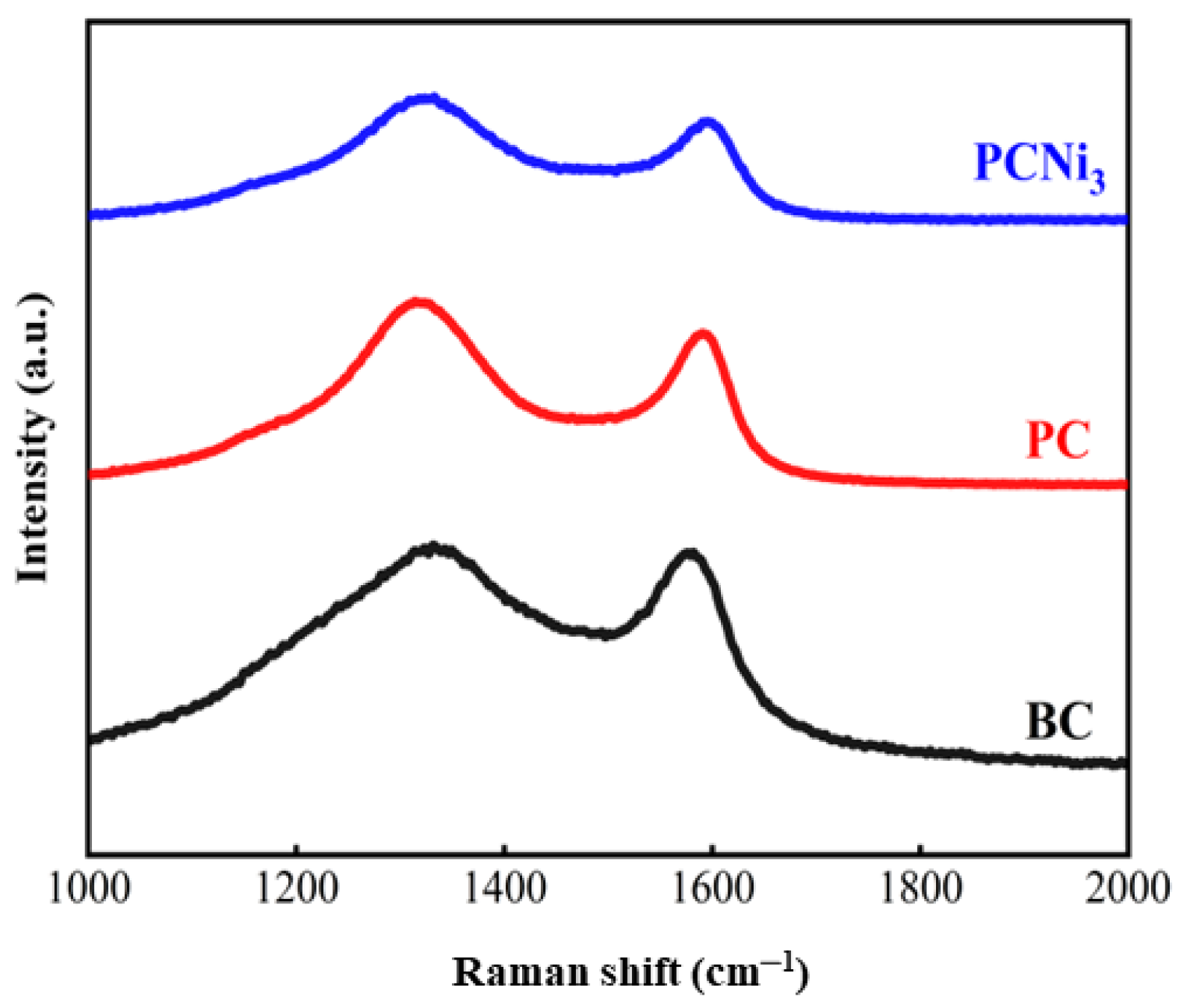
2.1. The Physicochemical Properties of Adsorbents
2.2. The Magnetic Properties of Adsorbents
2.3. Effect of Initial Solution pH on the Adsorption
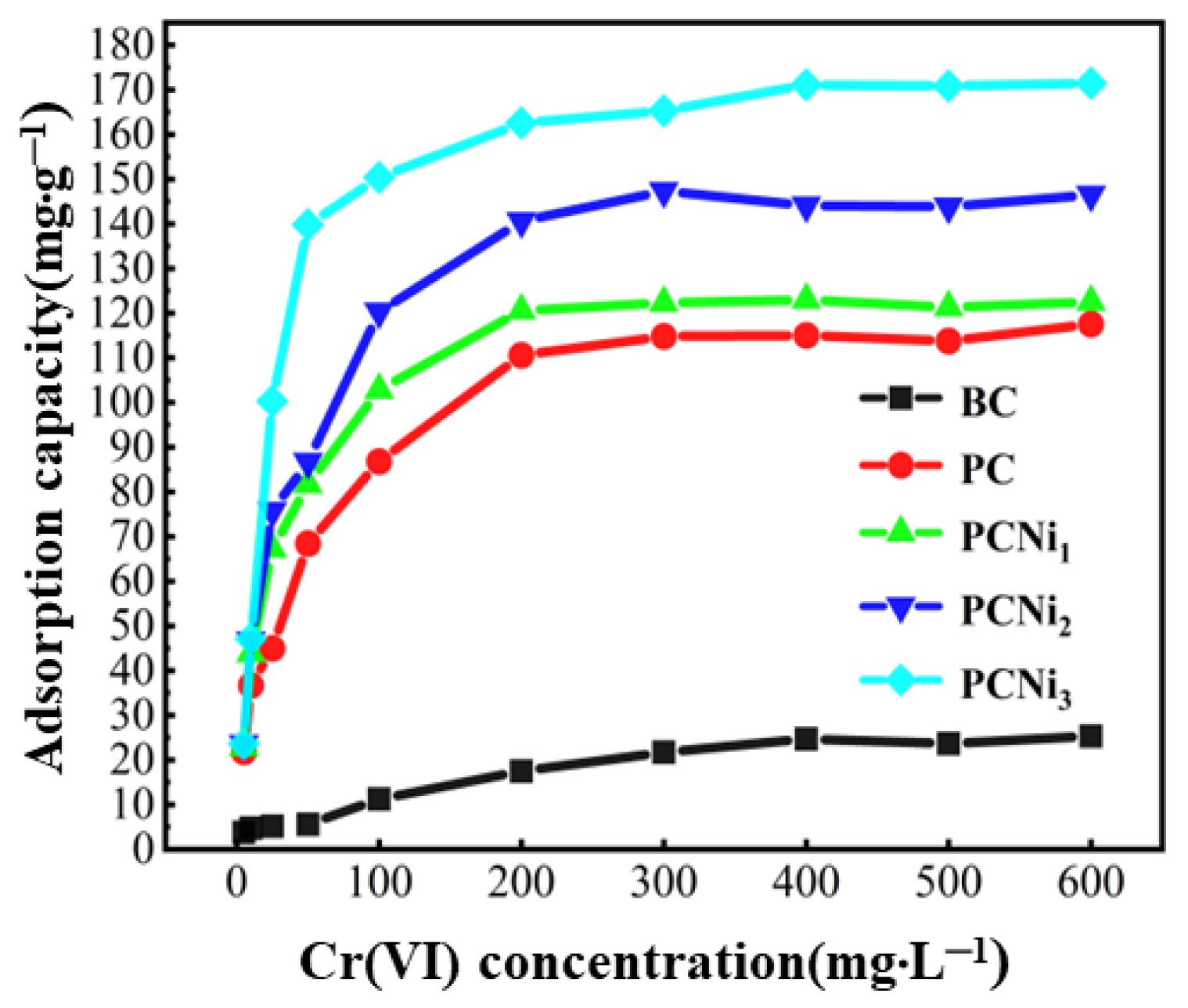
2.4. Effect of Initial Solution Concentration on Adsorption
2.5. Time Effect on Adsorption
2.6. Thermodynamic Investigation
2.7. Removal Mechanism
2.8. Comparison of Cr (VI) Adsorption Performances of Adsorbents
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syed, A.; Zeyad, M.T.; Shahid, M.; Elgorban, A.M.; Alkhulaifi, M.M.; Ansari, I.A. Heavy Metals Induced Modulations in Growth, Physiology, Cellular Viability, and Biofilm Formation of an Identified Bacterial Isolate. ACS Omega 2021, 6, 25076–25088. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Han, G.; Liu, J.; Zhang, S.; Zeng, J. Heavy Metal Accumulation and Source Apportionment in Urban River under Ecological Restoration: Relationships with Land Use and Risk Assessment Based on Monte Carlo Simulation. ACS Earth Space Chem. 2023, 7, 642–652. [Google Scholar] [CrossRef]
- Xia, S.; Liang, S.; Qin, Y.; Chen, W.; Xue, B.; Zhang, B.; Xu, G. Significant Improvement of Adsorption for Phosphate Removal by Lanthanum-Loaded Biochar. ACS Omega 2023, 8, 24853–24864. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Fan, J.; Wan, K.; Wang, G.; Xiao, Y.; Bo, W.; Gao, M.; Miao, Z. Calcium-Modified Fe3O4 Nanoparticles Encapsulated in Humic Acid for the Efficient Removal of Heavy Metals from Wastewater. Langmuir 2021, 37, 10994–11007. [Google Scholar] [CrossRef] [PubMed]
- Rafieyan, S.G.; Marahel, F.; Ghaedi, M.; Maleki, A. Application of Terminalia catappa wood-based activated carbon modified with CuO nanostructures coupled with H2O2 for the elimination of chemical oxygen demand in the gas refinery. J. Nanostruct. Chem. 2022, 12, 159–177. [Google Scholar] [CrossRef]
- Cui, P.; Liu, C.; Su, X.; Yang, Q.; Ge, L.; Huang, M.; Dang, F.; Wu, T.; Wang, Y. Atomically Dispersed Manganese on Biochar Derived from a Hyperaccumulator for Photocatalysis in Organic Pollution Remediation. Environ. Sci. Technol. 2022, 56, 8034–8042. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Zych, A.; Athanassiou, A. Biodegradable and Biobased Mulch Films: Highly Stretchable PLA Composites with Different Industrial Vegetable Waste. ACS Appl. Mater. Interfaces 2022, 14, 46920–46931. [Google Scholar] [CrossRef]
- Khan, Z.H.; Li, Z.; Gao, M.; Islam, S.; Xiao, L.; Qiu, W.; Song, Z. Simultaneous and efficient removal of Cd(II) and As(III) by a magnesium-manganese codoped biochar composite: Sorption performance and governing mechanisms. J. Environ. Chem. Eng. 2023, 11, 109919. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Yan, R.; Li, J.; Li, C.; Liang, S. Removal performance and mechanisms of aqueous Cr (VI) by biochar derived from waste hazelnut shell. Environ. Sci. Pollut. Res. 2023, 30, 97310–97318. [Google Scholar] [CrossRef]
- Grimm, A.; Chen, F.; dos Reis, G.S.; Dinh, V.M.; Khokarale, S.G.; Finell, M.; Mikkola, J.-P.; Hultberg, M.; Dotto, G.L.; Xiong, S. Cellulose Fiber Rejects as Raw Material for Integrated Production of Pleurotus spp. Mushrooms and Activated Biochar for Removal of Emerging Pollutants from Aqueous Media. ACS Omega 2023, 8, 5361–5376. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Q.; Wang, H.; Zhou, Z.; Li, N.; Pan, H.; Liu, Y.; Liu, X. Enhanced Adsorptive Removal of Tetracycline by Phosphomolybdic Acid-Modified Low-Temperature Sludge Biochar. Langmuir 2023, 40, 751–760. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, C.; Wu, H. Sustainable utilization of wetland biomass for activated carbon production: A review on recent advances in modification and activation methods. Sci. Total Environ. 2021, 790, 148214. [Google Scholar] [CrossRef]
- Feng, P.; Li, J.; Wang, H.; Xu, Z. Biomass-Based Activated Carbon and Activators: Preparation of Activated Carbon from Corncob by Chemical Activation with Biomass Pyrolysis Liquids. ACS Omega 2020, 5, 24064–24072. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A.; Zhang, K.; Lu, Y.; Nabi, I.; Naseem, F.; Ullah, H. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Yang, Z.; Gleisner, R.; Mann, D.H.; Xu, J.; Jiang, J.; Zhu, J.Y. Lignin Based Activated Carbon Using H3PO4 Activation. Polymers 2020, 12, 2829. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, H.; Han, L.; Yang, N.; Hu, B.; Qiu, M.; Zhong, X. Highly efficient removal of U(VI) by a novel biochar supported with FeS nanoparticles and chitosan composites. J. Mol. Liq. 2021, 327, 114807. [Google Scholar] [CrossRef]
- Yap, M.W.; Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater. J. Ind. Eng. Chem. 2017, 45, 287–295. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Li, C.; Yue, F.; Zheng, L. Properties and mechanism of Cr(VI) removal by a ZnCl2-modified sugarcane bagasse biochar–supported nanoscale iron sulfide composite. Environ. Sci. Pollut. Res. 2022, 30, 26889–26900. [Google Scholar] [CrossRef]
- Shen, Y. Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications. J. Mater. Chem. A 2015, 3, 13114–13188. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, N.; Qin, X.; Liu, X.; Shi, S.; Yan, M.; Liu, Q.; Liu, Z. Effect of Microstructure and Macro Size of Walnut Shell Chars on Their Electromagnetic Induction Heating Behavior. Ind. Eng. Chem. Res. 2023, 62, 9726–9734. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Tang, J.; He, J.; Sun, H. Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 2020, 241, 125044. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, J.; Ling, P.; Zhang, X.; Xu, K.; He, L.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Chen, M.; He, F.; Hu, D.; Bao, C.; Huang, Q. Broadened operating pH range for adsorption/reduction of aqueous Cr(VI) using biochar from directly treated jute (Corchorus capsularis L.) fibers by H3PO4. Chem. Eng. J. 2020, 381, 122739. [Google Scholar] [CrossRef]
- Yasdi, Y.; Rinaldi, R.; Anggraini, F.J.; Yulianti, T. Biochar from Oil Palm Frond to Reduce Fe Ions in Artificial Solution and Peat Water. Adv. Mater. Res. 2021, 1162, 49–56. [Google Scholar] [CrossRef]
- Deng, Z.; Deng, Q.; Wang, L.; Xiang, P.; Lin, J.; Murugadoss, V.; Song, G. Modifying coconut shell activated carbon for improved purification of benzene from volatile organic waste gas. Adv. Compos. Hybrid Mater. 2021, 4, 751–760. [Google Scholar] [CrossRef]
- Zeng, B.; Xu, W.; Khan, S.B.; Wang, Y.; Zhang, J.; Yang, J.; Su, X.; Lin, Z. Preparation of sludge biochar rich in carboxyl/hydroxyl groups by quenching process and its excellent adsorption performance for Cr(VI). Chemosphere 2021, 285, 131439. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L.-J. Metal Interactions at the Biochar-Water Interface: Energetics and Structure-Sorption Relationships Elucidated by Flow Adsorption Microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Tian, B.; Li, T.; Zhang, J.; Zhao, H. The preparation of amino-reinforced phosphorylated biochar for efficient uranium adsorption. J. Radioanal. Nucl. Chem. 2023, 332, 3305–3315. [Google Scholar] [CrossRef]
- Ma, L.; Du, Y.; Chen, S.; Du, D.; Ye, H.; Zhang, T.C. Highly efficient removal of Cr(VI) from aqueous solution by pinecone biochar supported nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1. Chemosphere 2022, 287, 132184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Wu, R.; Liu, Y.; Lin, X.; Kan, H.; Zheng, Y. Preparation of Acid- and Alkali-Modified Biochar for Removal of Methylene Blue Pigment. ACS Omega 2020, 5, 30906–30922. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Yang, H.; Hong, M.; Chen, S. Removal of Cr(VI) with nano-FeS and CMC-FeS and transport properties in porous media. Environ. Technol. 2020, 41, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Min, X.; Peng, T.; Zhao, F.; Ke, Y.; Wang, Y.; Jiang, G.; Xu, Q.; Wang, J. Mechanochemically Activated Microsized Zero-Valent Iron/Pyrite Composite for Effective Hexavalent Chromium Sequestration in Aqueous Solution. J. Chem. Eng. Data 2020, 65, 1936–1945. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Wang, G.; Yang, Z.; Xian, J.; Yang, Y.; Li, T.; Pu, Y.; Jia, Y.; Li, Y.; et al. Adsorption and reduction of Cr(VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105407. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Ma, J.; Ning, P. Fe(III) modified Egeria najas driven-biochar for highly improved reduction and adsorption performance of Cr(VI). Powder Technol. 2021, 388, 485–495. [Google Scholar] [CrossRef]
- Ma, R.; Yan, X.; Pu, X.; Fu, X.; Bai, L.; Du, Y.; Cheng, M.; Qian, J. An exploratory study on the aqueous Cr(VI) removal by the sulfate reducing sludge-based biochar. Sep. Purif. Technol. 2021, 276, 119314. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, X.; Wu, X.; Ke, C.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van der Bruggen, B. Simultaneous Removal of Trivalent Chromium and Hexavalent Chromium from Soil Using a Modified Bipolar Membrane Electrodialysis System. Environ. Sci. Technol. 2020, 54, 13304–13313. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, C.; Guan, Q.; He, L.; Zhou, H.; Xin, H.; He, M.; Zhang, X.; Liu, R. Efficient Anchoring of Cu(II)–Tetracycline Complex in Paper Mill Sludge Biochar-Limited Nanospace. ACS EST Water 2024, 4, 166–177. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Z.; Xie, H.; Liang, W.; Luo, J.; Dang, C.; Wang, A.; Hu, L.; Yu, X.; Cai, W. Removal of phenylarsonic acid compounds by porous nitrogen doped carbon: Experimental and DFT study. Appl. Surf. Sci. 2022, 606, 154859. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Nguyen, Q.T.; Nguyen, T.H.; Nguyen, T.B.L.; Nguyen, V.Q.; Bui, T.U.; Le Sy, H. Paper waste sludge derived-hydrochar modified by iron (III) chloride for effective removal of Cr(VI) from aqueous solution: Kinetic and isotherm studies. J. Water Process Eng. 2021, 39, 101877. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; He, H.; Zhang, Y.; Zhang, Y.; Yin, L.; Li, J.; Yang, S.; Li, S.; et al. Humic acid modified nano-ferrous sulfide enhances the removal efficiency of Cr(VI). Sep. Purif. Technol. 2020, 240, 116623. [Google Scholar] [CrossRef]
- Zhu, S.; Ho, S.-H.; Huang, X.; Wang, D.; Yang, F.; Wang, L.; Wang, C.; Cao, X.; Ma, F. Magnetic Nanoscale Zerovalent Iron Assisted Biochar: Interfacial Chemical Behaviors and Heavy Metals Remediation Performance. ACS Sustain. Chem. Eng. 2017, 5, 9673–9682. [Google Scholar] [CrossRef]
- Zhang, X. Study on the Adsorption Properties of Cr(VI) by Biochar with Different Treatments. Nat. Environ. Pollut. Technol. 2023, 22, 1563–1569. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.; Wang, Q. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shao, J.; Li, Z.; Yang, H.; Zhang, S.; Chen, H. Nano nickel embedded in N-doped CNTs-supported porous biochar for adsorption-reduction of hexavalent chromium. J. Hazard. Mater. 2021, 416, 125693. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.A.; Abdel-Wahhab, M.A. Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci. Technol. 2018, 75, 36–45. [Google Scholar] [CrossRef]
- Zaidi, A.; Wani, P.A.; Khan, M.S. (Eds.) Toxicity of Heavy Metals to Legumes and Bioremediation; Springer: Vienna, Austria, 2012. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, N.; Feng, C.; Gao, Y. Mechanisms of Cr(VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@LS-BC) using mass-balance and functional group expressions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 17–24. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, H.; Pang, Y.; Zhan, H.; Zeng, C. Iron modified chitosan/coconut shell activated carbon composite beads for Cr(VI) removal from aqueous solution. Int. J. Biol. Macromol. 2023, 224, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, A.; Goharrizi, B.A.; Shahriari, T.; Ahmadi, S. Adsorption of chromium (VI) and Acid Orange 7 on lemon peel biochar: A response surface methodology approach. Int. J. Environ. Sci. Technol. 2023, 20, 2939–2958. [Google Scholar] [CrossRef]
- Luo, X.; Du, H.; Zhang, X.; Yang, Y. Amine-functionalized magnetic biochars derived from invasive plants Alternanthera philoxeroides for enhanced efficient removal of Cr(VI): Performance, kinetics and mechanism studies. Environ. Sci. Pollut. Res. 2022, 29, 78092–78106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, R.; Pu, X.; Fu, X.; Ju, X.; Arif, M.; Yan, X.; Qian, J.; Liu, Y. The characterization of a novel magnetic biochar derived from sulfate-reducing sludge and its application for aqueous Cr(VI) removal through synergistic effects of adsorption and chemical reduction. Chemosphere 2022, 308, 136258. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, H.; Zeng, C.; Zhan, H.; Pang, Y. Removal of Cr(VI) from Wastewater Using Graphene Oxide Chitosan Microspheres Modified with α–FeO(OH). Materials 2022, 15, 4909. [Google Scholar] [CrossRef]











| Sample | Langmuir | Freundlich | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL | qm | R2 | KF | nF | R2 | qs | m | KS | R2 | |
| PC | 0.045 | 121.09 | 0.904 | 31.24 | 4.52 | 0.957 | 195.15 | 2.59 | 0.007 | 0.969 |
| PCNi3 | 0.390 | 166.89 | 0.890 | 87.01 | 8.54 | 0.971 | 235.90 | 4.23 | 0.154 | 0.993 |
| Sample | Pseudo-First Order | Pseudo-Second Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | q1 | R2 | k2 | q2 | R2 | α | β | R2 | |
| PC | 0.014 | 57.92 | 0.790 | 3.33 × 10−4 | 63.33 | 0.913 | 5.18 | 0.098 | 0.993 |
| PCNi3 | 0.007 | 132.08 | 0.976 | 5.59 × 10−5 | 152.97 | 0.997 | 3.72 | 0.035 | 0.986 |
| Sample | Intra-Particle Diffusion | |||||
|---|---|---|---|---|---|---|
| k1 | C1 | k2 | C2 | k3 | C3 | |
| PC | 7.366 | −6.018 | 3.421 | 47.11 | 1.193 | 98.58 |
| PCNi3 | 2.765 | 11.44 | 1.161 | 23.46 | 0.738 | 40.74 |
| Sample | ΔG (kJ·mol−1) | ΔH | ΔS | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 313 K | 318 K | (kJ·mol−1) | J·(mol·K)−1 | |
| PC | −2.365 | −1.911 | −2.006 | −1.735 | −1.628 | −10.03 | −26.41 |
| PCNi3 | −4.902 | −4.255 | −5.523 | −4.980 | −6.019 | 11.81 | 55.40 |
| Adsorbent | qmax (mg·g−1) | pH | Dosage | C0 (mg·L−1) | T (°C) | tcontact (h) | Refs. |
|---|---|---|---|---|---|---|---|
| Chitosan microspheres | 24.16 | 3.0 | 1 g·L−1 | 25 | 25 | 24 | [54] |
| Lemon peel | 41.65 | 2.0 | 0.2 g·L−1 | 50 | 25 | 2 | [55] |
| Alternanthera Philoxeroides | 42.47 | 2.0 | 0.05 g | 100 | 25 | 24 | [56] |
| Sulfate-reducing sludge | 58.56 | 3.0 | 0.8 g | 50 | 25 | 24 | [57] |
| Camellia oleifera shells | 64.49 | 3.0 | 1 g·L−1 | 25 | 25 | 48 | [58] |
| Chestnut shells | 143.51 | 2.0 | 0.02 g | 50 | 25 | 24 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Zhang, X.; Chen, M.; Rahman, S.T.; Li, X.; Wang, G. Enhancing Cr (VI) Adsorption of Chestnut Shell Biochar through H3PO4 Activation and Nickel Doping. Molecules 2024, 29, 2220. https://doi.org/10.3390/molecules29102220
Hu W, Zhang X, Chen M, Rahman ST, Li X, Wang G. Enhancing Cr (VI) Adsorption of Chestnut Shell Biochar through H3PO4 Activation and Nickel Doping. Molecules. 2024; 29(10):2220. https://doi.org/10.3390/molecules29102220
Chicago/Turabian StyleHu, Wen, Xiaojing Zhang, Ming Chen, Sheikh Tamjidur Rahman, Xin Li, and Geming Wang. 2024. "Enhancing Cr (VI) Adsorption of Chestnut Shell Biochar through H3PO4 Activation and Nickel Doping" Molecules 29, no. 10: 2220. https://doi.org/10.3390/molecules29102220




