Effect of the Addition of Dandelion (Taraxacum officinale) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Assessment of Antioxidant Properties of Water Extract from Dandelion Leaves
2.2. Assessment of Physicochemical Parameters during Ripening
2.3. Assessment of Protein Profile
2.4. Assessment of Antiradical Potential of Raw-Ripening Pork Sausage
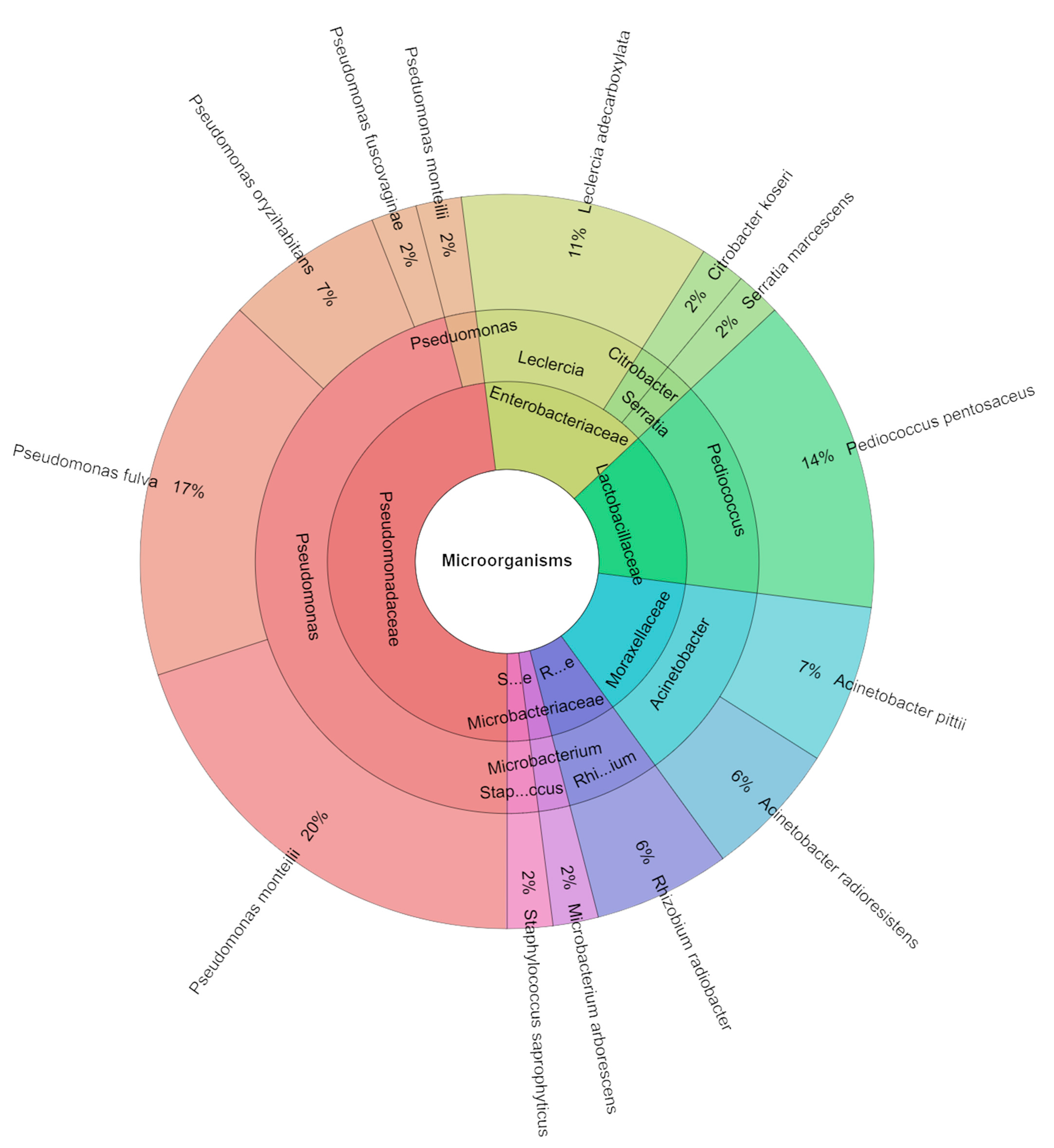
2.5. Microbiological Profile
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Determination of Antioxidant Capacity of Water Extract from Dandelion Leaves
3.2.1. Antioxidant Activity
3.2.2. Determination of Total Phenolic Content (TPC)
3.2.3. Determination of Total Flavonoids
3.2.4. Extract Preparation for Vitamin C Content Determination
3.2.5. LC-MS Analysis
4. Meat Product Analysis
4.1. Preparation of the Meat Product
4.2. Physicochemical Parameters
4.2.1. Total Acidity
4.2.2. Oxidation-Reduction Potential
4.2.3. Water Activity
4.3. TBARS Level
4.4. Electrophoretic Separation
4.5. Evaluation of Antiradical Activity
4.5.1. Obtaining the Extracts and Peptides
4.5.2. Antiradical Activity
4.6. Microbiological Analysis
4.6.1. Identification of Microorganisms Using Mass Spectrometry
4.6.2. Preparing the MALDI-TOF Matrix Solution
4.6.3. Identification of Microorganisms
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Xie, Y.; Cao, H.; Yang, H.; Chen, X.; Xiao, J. Fetal bovine serum influences the stability and bioactivity of resveratrol analogues: A polyphenol-protein interaction approach. Food Chem. 2017, 219, 321–328. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Zhou, L.; Elias, R.J. Antioxidant and pro-oxidant activity of (−)-epigallocatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef]
- Decker, E.A. Phenolics: Prooxidants or Antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef]
- González-Castejón, M.; García-Carrasco, B.; Fernández-Dacosta, R.; Dávalos, A.; Rodriguez-Casado, A. Reduction of Adipogenesis and Lipid Accumulation by Taraxacum officinale (Dandelion) Extracts in 3T3L1 Adipocytes: An in vitro Study. Phytother. Res. 2013, 28, 745–752. [Google Scholar] [CrossRef]
- Lis, B.; Grabek-Lejko, D. Mniszek lekarski (Taraxacum officinale)—Potencjalne właściwości prozdrowotne. Nauka Przyr. Technol. 2016, 10, 37. [Google Scholar]
- Sajad, S.; Ahmad, S.R.; Irshad, S.; Qureshi, A.I.; Fayaz, A.; Mehraj, F.; Jalal, H. Influence of the aqueous extract of dandelion (Taraxacum officinale) powder on the quality of chicken meat loaves. J. Entomol. Zool. Stud. 2020, 8, 1579–1582. [Google Scholar]
- Choi, Y.J.; Park, K.S.; Jung, I.C. Quality characteristics of ground pork meat containing hot water extract from dandelion (Taraxacum officinale). J. East. Asian Soc. Diet. Life 2015, 25, 651–659. [Google Scholar] [CrossRef]
- Kęska, P.; Wójciak, K.M.; Stasiak, D.M. Influence of sonication and Taraxacum officinale addition on the antioxidant and anti-ACE activity of protein extracts from sous vide beef marinated with sour milk and after in vitro digestion. Molecules 2020, 25, 4692. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.I.; De Smet, S. The recommendation to limit or avoid consumption of processed meat is justified because of the association with the incidence of colorectal cancer and justifies the use of alternatives for nitrite in meat processing. Nitric Oxide 2010, 23, 150–151. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Ferysiuk, K.; Kęska, P.; Materska, M.; Chilczuk, B.; Trząskowska, M.; Kruk, M.; Kołożyn-Krajewska, D.; Domínguez, R. Reduction of Nitrite in Canned Pork through the Application of Black Currant (Ribes nigrum L.) Leaves Extract. Molecules 2023, 28, 1749. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M.; Materska, M.; Chilczuk, B.; Pabich, M. Modification of lipid oxidation and antioxidant capacity in canned refrigerated pork with a nitrite content reduced by half and addition of sweet pepper extract. LWT Food Sci. Technol. 2020, 118, 108738. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M.; Trząskowska, M. Fortification of low-nitrite canned pork with willow herb (Epilobium angustifolium L.). Int. J. Food Sci. Technol. 2022, 57, 4194–4210. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Myoprotein–phytophenol interaction: Implications for muscle food structure-forming properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2801–2824. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, J.-R.; Liu, X.-M.; Zhu, M.-J. Effect of microencapsulated process on stability of mulberry polyphenol and oxidation property of dried minced pork slices during heat processing and storage. LWT Food Sci. Technol. 2019, 100, 62–68. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, Y.; Ma, G.; Song, H.; Li, H.; Wang, Z.; Xiao, S. Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. 2011, 88, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Xing Tang, K.; Shi, T.; Gänzle, M. Effect of starter cultures on taste-active amino acids and survival of pathogenic Escherichia coli in dry fermented beef sausages. Eur. Food Res. Technol. 2018, 244, 2203–2212. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Chen, C.; Xie, T.; Li, P. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Res. Int. 2020, 135, 109247. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Feng, M.; Sun, J. Influence of mixed starters on the degradation of proteins and the formation of peptides with antioxidant activities in dry fermented sausages. Food Control 2021, 123, 107743. [Google Scholar] [CrossRef]
- Lücke, F.K. European Products. In Handbook of Fermented Meat and Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 287–292. [Google Scholar]
- Lorenzo, J.M.; Gόmez, M.; Fonseca, S. Effect of commercial starter cultures on physicochemical characteristics, microbial counts and free fatty acid composition of dry-cured foal sausage. Food Control 2014, 46, 382–389. [Google Scholar] [CrossRef]
- Okoń, A.; Szymański, P.; Dolatowski, Z.J. Wpływ serwatki kwasowej na jakość fizykochemiczną i stabilność barwy fermentowanych kiełbas ekologicznych. Żywność. Nauka. Technologia. Jakość 2019, 26, 135–147. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P.; Gazda, P.; Siłka, Ł.; Kołożyn-Krajewska, D. Influence of LAB fermentation on the color stability and oxidative changes in dry-cured meat. Appl. Sci. 2022, 12, 11736. [Google Scholar] [CrossRef]
- Flores, J.; Marcus, J.R.; Nieto, P.; Navarro, J.L.; Lorenzo, P. Effect of processing conditions on proteolysis and taste of dry-cured sausages. Z. Lebensm. Unters. Forsch. A 1997, 204, 168–172. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J. Effect of acid whey on nitrosylmyoglobin concentration in uncured fermented sausage. LWT Food Sci. Technol. 2015, 64, 713–719. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Jackson-Davis, A.L.; Myers, K.L.; Lavieri, N.A. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J.; Kołożyn-Krajewska, D.; Trząskowska, M. The effect of the Lactobacillus Casei Lock 0900 probiotic strain on the quality of dry-fermented sausage during chilling storage. J. Food Qual. 2012, 35, 353–365. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Jaworska, D.; Kołożyn-Krajewska, D.; Dolatowski, Z.J.; Jachacz-Jówko, L. The effect of LAB as probiotic starter culture and green tea extract addition on dry fermented pork loins quality. Biomed. Res. Int. 2015, 2015, 452757. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, Q.; Li, F.; Zheng, D.; Kong, B. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Control. 2016, 68, 358–366. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; García, C.; Rondán, J.J.; Núñez, F. Effects of preservative agents on quality attributes of dry-cured fermented sausages. Foods 2020, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ockerman, H.W.; Basu, L. Production and consumption of fermented meat products. In Handbook of Fermented Meat and Poultry; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 7–11. [Google Scholar]
- Kononiuk, A.D.; Karwowska, M. Porównanie zmian fizykochemicznych i proteolitycznych zachodzących w kiełbasach surowo dojrzewających z mięsa wołowego i mięsa z daniela podczas ich przechowywania. Żywność. Nauka. Technologia. Jakość 2016, 26, 137–154. [Google Scholar] [CrossRef]
- Candogan, K.; Wardlaw, F.B.; Acton, J.C. Effect of starter culture on proteolytic changes during processing of fermented beef sausages. Food Chem. 2009, 116, 731–737. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Aristoy, C.; Toldrá, F. The use of label-free mass spectrometry for relative quantification of sarcoplasmic proteins during the processing of dry-cured ham. Food Chem. 2016, 196, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gao, Y.; Solangi, I.; Liu, S.; Zhu, J. Effects of tea polyphenols on the conformational, functional, and morphological characteristics of beef myofibrillar proteins. LWT Food Sci. Technol. 2022, 154, 112596. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Glucose oxidase promotes gallic acid-myofibrillar protein interaction and thermal gelation. Food Chem. 2019, 293, 529–536. [Google Scholar] [CrossRef]
- Cheng, J.; Xiang, R.; Tang, D.; Zhu, M.; Liu, X. Regulation of protein oxidation in Cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2021, 175, 108422. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, M.; Kolanek, M. Flavonoids and their properties to form chelate complexes. Biotechnol. Food Sci. 2012, 76, 35–41. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Ge, Q.; Pei, H.; Liu, R.; Chen, L.; Gao, X.; Gu, Y.; Hou, Q.; Yin, Y.; Yu, H.; Wu, M.; et al. Effects of Lactobacillus plantarum NJAU-01 from Jinhua ham on the quality of dry-cured fermented sausage. LWT Food Sci. Technol. 2019, 101, 513–518. [Google Scholar] [CrossRef]
- Weiss, J.; Loeffler, M.; Terjung, N. The antimicrobial paradox: Why preservatives lose activity in foods. Curr. Opin. Food Sci. 2015, 4, 69–75. [Google Scholar] [CrossRef]
- Franciosa, I.; Alessandria, V.; Dolci, P.; Rantsiou, K.; Cocolin, L. Sausage fermentation and starter cultures in the era of molecular biology methods. Int. J. Food Microbiol. 2018, 279, 26–32. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef]
- Yim, D.-G.; Ali, M.; Nam, K.-C. Comparison of Meat Quality Traits in Salami Added by Nitrate-free Salts or Nitrate Pickling Salt during Ripening. Food Sci. Anim. Resour. 2020, 40, 11–20. [Google Scholar] [CrossRef]
- Honikel, K.-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Settanni, L.; Barbaccia, P.; Bonanno, A.; Ponte, M.; Di Gerlando, R.; Franciosi, E.; Di Grigoli, A.; Gaglio, R. Evolution of indigenous starter microorganisms and physicochemical parameters in spontaneously fermented beef, horse, wild boar and pork salamis produced under controlled conditions. Food Microbiol. 2020, 87, 103385. [Google Scholar] [CrossRef]
- Quijada, N.M.; De Filippis, F.; Sanz, J.J.; García-Fernández, M.d.C.; Rodríguez-Lázaro, D.; Ercolini, D.; Hernández, M. Different Lactobacillus populations dominate in “Chorizo de León” manufacturing performed in different production plants. Food Microbiol. 2018, 70, 94–102. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Ranucci, D.; Miraglia, D.; Cioffi, A. Use of starter cultures of dairy origin in the production of Salame nostrano, an Italian dry-cured sausage. Meat Sci. 2008, 78, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P.; Hertel, C. New Developments in Meat Starter Cultures. Meat Sci. 1998, 49, S125–S138. [Google Scholar] [CrossRef]
- Aquilanti, L.; Santarelli, S.; Silvestri, G.; Osimani, A.; Petruzzelli, A.; Clementi, F. The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 2007, 120, 136–145. [Google Scholar] [CrossRef]
- Cocolin, L.; Dolci, P.; Rantsiou, K. Biodiversity and dynamics of meat fermentations: The contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011, 89, 296–302. [Google Scholar] [CrossRef]
- Talon, R.; Leroy, S. Diversity and safety hazards of bacteria involved in meat fermentations. Meat Sci. 2011, 89, 303–309. [Google Scholar] [CrossRef]
- Marty, E.; Buchs, J.; Eugster-Meier, E.; Lacroix, C.; Meile, L. Identification of staphylococci and dominant lactic acid bacteria in spontaneously fermented Swiss meat products using PCR–RFLP. Food Microbiol. 2012, 29, 157–166. [Google Scholar] [CrossRef]
- Chaillou, S.; Daty, M.; Baraige, F.; Dudez, A.M.; Anglade, P.; Jones, R.; Alpert, C.A.; Champomier-Verges, M.C.; Zagorec, M. Intraspecies genomic diversity and natural population structure of the meat-borne lactic acid bacterium Lactobacillus sakei. Appl. Environ. Microbiol. 2009, 75, 970–980. [Google Scholar] [CrossRef]
- Di Cagno, R.; Lòpez, C.C.; Tofalo, R.; Gallo, G.; De Angelis, M.; Paparella, A.; Hammes, W.P.; Gobbetti, M. Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of three Italian PDO fermented sausages. Meat Sci. 2008, 79, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef] [PubMed]
- Werlang, G.O.; Vieira, T.R.; Cardoso, M.; Costa, E. de F. Application of a predictive microbiological model for estimation of Salmonella behavior throughout the manufacturing process of salami in environmental conditions of small-scale Brazilian manufacturers. Microb. Risk Anal. 2021, 19, 100177. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. FreeRadicalBio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Wu, X.; Diao, Y.; Sun, C.; Yang, J.; Wang, Y.; Sun, S. Fluorimetric determination of ascorbic acid with o-phenylenediamine. Talanta 2003, 59, 95–99. [Google Scholar] [CrossRef]
- PN-ISO 2917:2001; Mięso i Przetwory Mięsne—Pomiar pH—Metoda Odwoławcza. Polish Committee for Standardization: Warszawa, Poland, 2013. (In Polish)
- Nam, K.C.; Ahn, D.U. Effects of Ascorbic Acid and Antioxidants on the Color of Irradiated Ground Beef. J. Food Sci. 2003, 68, 1686–1690. [Google Scholar] [CrossRef]
- Fan, X.-J.; Liu, S.-Z.; Li, H.-H.; Feng, J.-T.; Zhang, X.; Yan, H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019, 147, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nat. Cell Biol. 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Identification of small troponin T peptides generated in dry-cured ham. Food Chem. 2010, 123, 691–697. [Google Scholar] [CrossRef]
- Jung, S.; Choe, J.C.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formmosanum Kitam by liquid chromatography- tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef]
- Diaz, M.I.; Barros, L.; Alves, R.C.; Oliveira, M.B.; Santos-Buelga, C.; Ferreira, I. Nutritional composition, antioxidant activity and phenolic compounds of wild Taraxacum sect. Ruderalia. Food Res. Int. 2014, 56, 266–271. [Google Scholar] [CrossRef]
- Miłek, M.; Marcincakova, D.; Legath, J. Polyphenols content, antioxidant activity and cytotoxic assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum genus): A review of chemical constituents and pharmacological effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]






| Analyzed Parameters | |
|---|---|
| Extraction yield (%) | 30.61 ± 0.11 |
| Vitamin C (mg/100 g) | 5.39 ± 0.01 |
| DPPH (EC50, μg/mL) | 68.4 ± 0.15 |
| ABTS (EC50, μg/mL) | 36.5 ± 0.05 |
| Total phenolic content (mg gallic acid/g) | 303.2 ± 4.7 |
| Flavonoids content (mg quercetin/g) | 18.06 ± 0.14 |
| No | Rt | Name * | Molecular Formula | Mobs | Mteor | m/z | Diff (ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 1.274 | Neochlorogenic acid | C16H18O9 | 354.0958 | 354.0951 | 355.1028 | 1.93 |
| 2 | 1.307 | Caffeoyltartaric acid | C13H12O9 | 312.0484 | 312.0481 | 313.0556 | 0.79 |
| 3 | 1.307 | 7-hydroxycoumarin | C9H6O3 | 162.0317 | 162.0317 | 163.039 | 0.23 |
| 4 | 1.623 | Cichoriin | C15H16O9 | 340.08 | 340.0794 | 341.0874 | 1.6 |
| 5 | 2.772 | Esculetin | C9H6O4 | 178.0274 | 178.0267 | 179.0347 | 4.47 |
| 6 | 3.288 | Dihydrosyringin | C17H26O9 | 374.1586 | 374.1577 | 375.1690 | 2.34 |
| 7 | 3.738 | Taraxafolin | C11H14O5 | 226.0858 | 226.0841 | 227.093 | 7.39 |
| 8 | 3.821 | Quercetin-3-O-Ara-Glc | C26H28O16 | 596.1384 | 596.1377 | 597.1455 | 1.19 |
| 9 | 3.855 | Quercetin-3,4′-di-Glc | C27H30O17 | 626.1522 | 626.1563 | 627.1578 | 6.21 |
| 10 | 3.888 | Quercetin-3-(malonyl-Glc)-Glc | C30H32O20 | 712.1501 | 712.1487 | 713.1567 | 2.03 |
| 11 | 4.021 | Caffeoyl malic acid | C13H12O8 | 296.0551 | 296.0532 | 297.0623 | 6.27 |
| 12 | 4.337 | Scopoletin | C10H8O4 | 192.0427 | 192.0423 | 193.0499 | 2.26 |
| 13 | 4.371 | Luteolin 3′,7-O-di-Glc | C27H30O16 | 610.1534 | 610.1523 | 611.1635 | 1.22 |
| 14 | 4.504 | Quercetin-3-O-Ara | C20H18O11 | 434.0871 | 434.0849 | 457.0769 | 5.15 |
| 15 | 4.504 | Chlorogenic acid | C16H18O9 | 354.0954 | 354.0951 | 355.1026 | 0.78 |
| 16 | 4.504 | L-chicoric acid | C22H18O12 | 474.0806 | 474.0798 | 475.0878 | 1.59 |
| 17 | 4.851 | Luteolin 7-O-Rhamnoside | C27H30O15 | 594.1585 | 594.1585 | 595.1652 | −0.87 |
| 18 | 4.903 | 3,5-di-O-caffeoylquinic acid | C25H24O12 | 516.1282 | 516.1023 | 517.1352 | 2.12 |
| 19 | 4.947 | Luteolin 7-O-Glc | C21H20O11 | 448.1012 | 448.1006 | 449.1085 | 1.51 |
| 20 | 5.268 | Rosmarinic acid | C18H16O8 | 360.084 | 360.0845 | 3383.0742 | −1.33 |
| 21 | 6.073 | Luteolin | C15H10O6 | 286.0456 | 286.0477 | 287.0527 | −7.45 |
| Parameter | Time [Day] | P_150 | P_80 | P_80_M05 | P_80_M1 |
|---|---|---|---|---|---|
| pH | 1 | 6.52 ± 0.02 Aab | 6.50 ± 0.01 Aa | 6.49 ± 0.03 Aa | 6.55 ± 0.01 Ab |
| 21 | 5.43 ± 0.05 Ba | 5.44 ± 0.04 BCa | 5.31 ± 0.01 Bb | 5.26 ± 0.01 Bc | |
| 51 | 5.44 ± 0.02 Ba | 5.42 ± 0.01 Ba | 5.32 ± 0.03 Bb | 5.28 ± 0.01 Cc | |
| 81 | 5.46 ± 0.03 Ba | 5.47 ± 0.03 Ca | 5.38 ± 0.02 Cb | 5.34 ± 0.01 Db | |
| ORP [mV] | 1 | 345.60 ± 1.21 Aa | 339.60 ± 1.00 Ab | 349.33 ± 1.49 Ac | 358.50 ± 2.82 Ad |
| 21 | 367.8 ± 4.42 Ba | 353.58 ± 13.65 Abb | 355.62 ± 6.99 Aab | 361.84 ± 5.58 ABab | |
| 51 | 385.97 ± 10.52 Ca | 367.75 ± 11.03 BCb | 365.17 ± 6.01 Bb | 366.65 ± 7.30 Bb | |
| 81 | 387.90 ± 1.53 Ca | 369.65 ± 7.55 Cb | 377.45 ± 3.77 Cc | 380.90 ± 1.20 Cc | |
| aw | 1 | 0.966 ± 0.003 Aa | 0.965 ± 0.002 Aa | 0.968 ± 0.001 Aab | 0.969 ± 0.001 Ab |
| 21 | 0.877 ± 0.007 Ba | 0.878 ± 0.008 Ba | 0.878 ± 0.002 Bab | 0.889 ± 0.007 Bb | |
| 51 | 0.871 ± 0.011 Ca | 0.883 ± 0.002 Bb | 0.879 ± 0.009 Bb | 0.884 ± 0.008 BCb | |
| 81 | 0.863 ± 0.003 BCa | 0.867 ± 0.006 Ca | 0.873 ± 0.006 Ba | 0.875 ± 0.010 Ca |
| Lp. | P0_150 | P0_80 | P0_80_M05 | P0_80_M1 | P90_150 | P90_80 | P90_80_M05 | P90_80_M1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | MW kDa | Vol *103 | |
| 1 | - | - | - | - | - | - | - | - | 147.5 | 240 | 170.4 | 356 | 174.8 | 258 | 203.8 | 249 |
| 2 | 131.0 | 168 | 135.5 | 136 | - | - | 137.8 | 115 | - | - | - | - | - | - | - | - |
| 3 | 76.6 | 169 | 80.8 | 172 | 83.4 | 89 | 79.1 | 145 | 87.1 | 106 | 90.9 | 123 | 93.8 | 106 | 96.9 | 169 |
| 4 | 63.9 | 816 | 65.0 | 815 | 67.2 | 627 | 67.2 | 560 | 68.9 | 693 | 70.7 | 712 | 71.9 | 724 | 75.0 | 617 |
| 5 | 57.7 | 1048 | 58.2 | 911 | 59.7 | 970 | 60.2 | 869 | 61.8 | 958 | 63.3 | 866 | 65.0 | 885 | 67.2 | 758 |
| 6 | 48.0 | 170 | 48.7 | 149 | 49.3 | 143 | 50.0 | 149 | 50.9 | 167 | 52.6 | 124 | 53.5 | 155 | 55.8 | 125 |
| 7 | 36.1 | 1867 | 37.0 | 1635 | 37.0 | 1769 | 37.8 | 1528 | 38.0 | 1683 | 39.8 | 1621 | 40.1 | 1679 | 42.0 | 1418 |
| 8 | 27.9 | 362 | 27.6 | 478 | 28.3 | 466 | 28.3 | 394 | 28.7 | 272 | 28.9 | 502 | 26.6 | 342 | 30.1 | 418 |
| 9 | 25.9 | 572 | 25.9 | 390 | 26.5 | 557 | 26.5 | 397 | 27 | 518 | 27.2 | 394 | 27.9 | 469 | 28.3 | 371 |
| 10 | 23.7 | 330 | 19.6 | 155 | 24.6 | 338 | 24.1 | 168 | 25.1 | 323 | 24.6 | 337 | 25.7 | 256 | 25.8 | 291 |
| 11 | - | - | - | - | - | - | 19.9 | 160 | - | - | 20.5 | 200 | 21.1 | 66 | 21.5 | 175 |
| 12 | 17.9 | 186 | 17.8 | 158 | - | - | 18.1 | 138 | - | - | 18.2 | 257 | - | - | 18.6 | 189 |
| 13 | 15.8 | 275 | 16.0 | 288 | 16.1 | 459 | 16.3 | 245 | 16.4 | 244 | 16.7 | 319 | 16.8 | 357 | 17.0 | 274 |
| 14 | 13.2 | 294 | 12.7 | 1490 | 13.6 | 814 | 13.1 | 1467 | 14.0 | 575 | 13.8 | 1532 | 14.3 | 661 | 14.4 | 684 |
| 15 | 10.6 | 340 | - | - | 11.0 | 754 | - | - | 11.2 | 682 | - | - | 11.3 | 981 | 10.5 | 454 |
| 16 | 10.0 | 195 | 10.0 | 442 | 10.0 | 417 | 10.0 | 483 | 10.0 | 328 | 10.2 | 470 | - | - | - | - |
| Parameter | Time [Day] | P_150 | P_80 | P_80_M05 | P_80_M1 |
|---|---|---|---|---|---|
| ABTSA-E | 1 | 33.33 ± 4.03 Aa | 39.90 ± 2.56 Ab | 35.24 ± 0.10 Aa | 37.24 ± 1.23 Aab |
| 81 | 45.55 ± 2.64 Ba | 47.79 ± 2.84 Ba | 52.36 ± 2.41 Bb | 59.98 ± 1.14 Bc | |
| ABTSW-E | 1 | 41.85 ± 1.49 Aa | 33.54 ± 2.92 Ab | 41.92 ± 3.81 Aa | 43.54 ± 2.22 Aa |
| 81 | 16.76 ± 1.03 Ba | 17.88 ± 0.62 Ba | 22.44 ± 0.72 Bb | 23.29 ± 1.03 Bb | |
| ABTSPEP | 1 | 18.52 ± 1.20 Aa | 10.44 ± 1.09 Ab | 23.65 ± 1.80 Ac | 28.17 ± 1.60 Ad |
| 81 | 17.85 ± 3.12 Aa | 10.55 1.16 Ab | 15.07 ± 2.78 Ba | 21.90 ± 2.75 Bc |
| Sample | Total Viable Count | Lactic Acid Bacteria | Coliforms Bacteria |
|---|---|---|---|
| 1 | 3.58 | 3.85 | 2.95 |
| 2 | 3.60 | 3.81 | 3.00 |
| 3 | 3.45 | 4.00 | 3.26 |
| 4 | 3.72 | 4.00 | 3.08 |
| 5 | 3.68 | 4.04 | 3.30 |
| 6 | 3.68 | 3.85 | 3.30 |
| 7 | 3.60 | 3.78 | 3.30 |
| 8 | 3.51 | 3.90 | 3.15 |
| 9 | 3.56 | 3.95 | 3.20 |
| 10 | 3.30 | 3.90 | 2.95 |
| Sample | Total Viable Count | Lactic Acid Bacteria | Coliforms Bacteria |
|---|---|---|---|
| 1 | 2.82 | 4.00 | ND |
| 2 | 3.15 | 4.04 | ND |
| 3 | 3.20 | 4.18 | ND |
| 4 | 3.26 | 4.04 | ND |
| 5 | 3.30 | 4.00 | ND |
| 6 | 3.20 | 4.00 | ND |
| 7 | 3.00 | 3.90 | ND |
| 8 | 3.20 | 3.95 | ND |
| 9 | 3.18 | 3.95 | ND |
| 10 | 3.60 | 4.04 | ND |
| Sample | Total Viable Count | Lactic Acid Bacteria | Coliforms Bacteria |
|---|---|---|---|
| 1 | 2.95 | 3.85 | ND |
| 2 | 3.30 | 3.90 | ND |
| 3 | 3.38 | 4.00 | ND |
| 4 | 3.08 | 3.95 | ND |
| 5 | 2.90 | 4.04 | ND |
| 6 | 2.86 | 3.68 | 3.00 |
| 7 | 2.98 | 3.90 | 2.78 |
| 8 | 2.88 | 3.93 | 3.08 |
| 9 | 3.11 | 4.04 | 2.90 |
| 10 | 3.56 | 3.88 | 3.00 |
| Sample | Total Viable Count | Lactic Acid Bacteria | Coliforms Bacteria |
|---|---|---|---|
| 1 | 3.30 | 4.00 | ND |
| 2 | 3.26 | 3.85 | ND |
| 3 | 3.30 | 4.04 | ND |
| 4 | 2.85 | 4.04 | ND |
| 5 | 3.28 | 3.95 | ND |
| 6 | 3.26 | 3.70 | 2.78 |
| 7 | 3.15 | 3.90 | 2.90 |
| 8 | 2.61 | 3.95 | 2.85 |
| 9 | 3.08 | 3.78 | 3.30 |
| 10 | 3.04 | 3.85 | 3.18 |
| Parameter | Time [Day] | P_150 | P_80 | P_80_M05 | P_80_M1 |
|---|---|---|---|---|---|
| DPPHA-E | 1 | 58.48 ± 3.77 Aa | 60.04 ± 0.92 Aa | 74.07 ± 1.66 Ab | 81.01 ± 0.82 Ac |
| 81 | 55.59 ± 1.38 Ba | 56.36 ± 1.96 Ba | 70.01 ± 2.31 Bb | 84.44 ± 1.57 Bc | |
| DPPHW-E | 1 | 14.83 ± 1.75 Aa | 13.66 0.41 Aa | 17.66 ± 0.60 Ab | 25.39 ± 0.53 Ac |
| 81 | 11.71 ± 0.21 Ba | 13.90 ± 0.20 Ab | 16.63 ± 1.62 Ac | 18.83 ± 0.20 Bc | |
| DPPHPEP | 1 | 15.92 ± 1.97 Aa | 16.72 ± 2.73 Aa | 22.58 ± 1.03 Ab | 24.53 ± 0.65 Ac |
| 81 | 12.28 ± 1.92 Ba | 15.76 ± 1.54 Ab | 18.32 ± 1.52 Bb | 24.25 ± 1.71 Bc |
| Nitrite (mg/kg) | Dandelion (%) | |
|---|---|---|
| P_150 | 150 | - |
| P_80 | 80 | - |
| P_80_M05 | 80 | 0.5 |
| P_80_M1 | 80 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, K.; Materska, M.; Pełka, A.; Michalska, A.; Małecka-Massalska, T.; Kačániová, M.; Čmiková, N.; Słowiński, M. Effect of the Addition of Dandelion (Taraxacum officinale) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage. Molecules 2024, 29, 2249. https://doi.org/10.3390/molecules29102249
Wójciak K, Materska M, Pełka A, Michalska A, Małecka-Massalska T, Kačániová M, Čmiková N, Słowiński M. Effect of the Addition of Dandelion (Taraxacum officinale) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage. Molecules. 2024; 29(10):2249. https://doi.org/10.3390/molecules29102249
Chicago/Turabian StyleWójciak, Karolina, Małgorzata Materska, Arkadiusz Pełka, Agata Michalska, Teresa Małecka-Massalska, Miroslava Kačániová, Natália Čmiková, and Mirosław Słowiński. 2024. "Effect of the Addition of Dandelion (Taraxacum officinale) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage" Molecules 29, no. 10: 2249. https://doi.org/10.3390/molecules29102249







