Molecular Self-Assembly and Adsorption Structure of 2,2′-Dipyrimidyl Disulfides on Au(111) Surfaces
Abstract
:1. Introduction
2. Results and Discussion
2.1. Concentration Effect on the Formation of 2PymS SAMs on Au(111) from DPymDS
2.2. The Formation of 2PymS SAMs on Au(111) from DPymDS at Different pHs
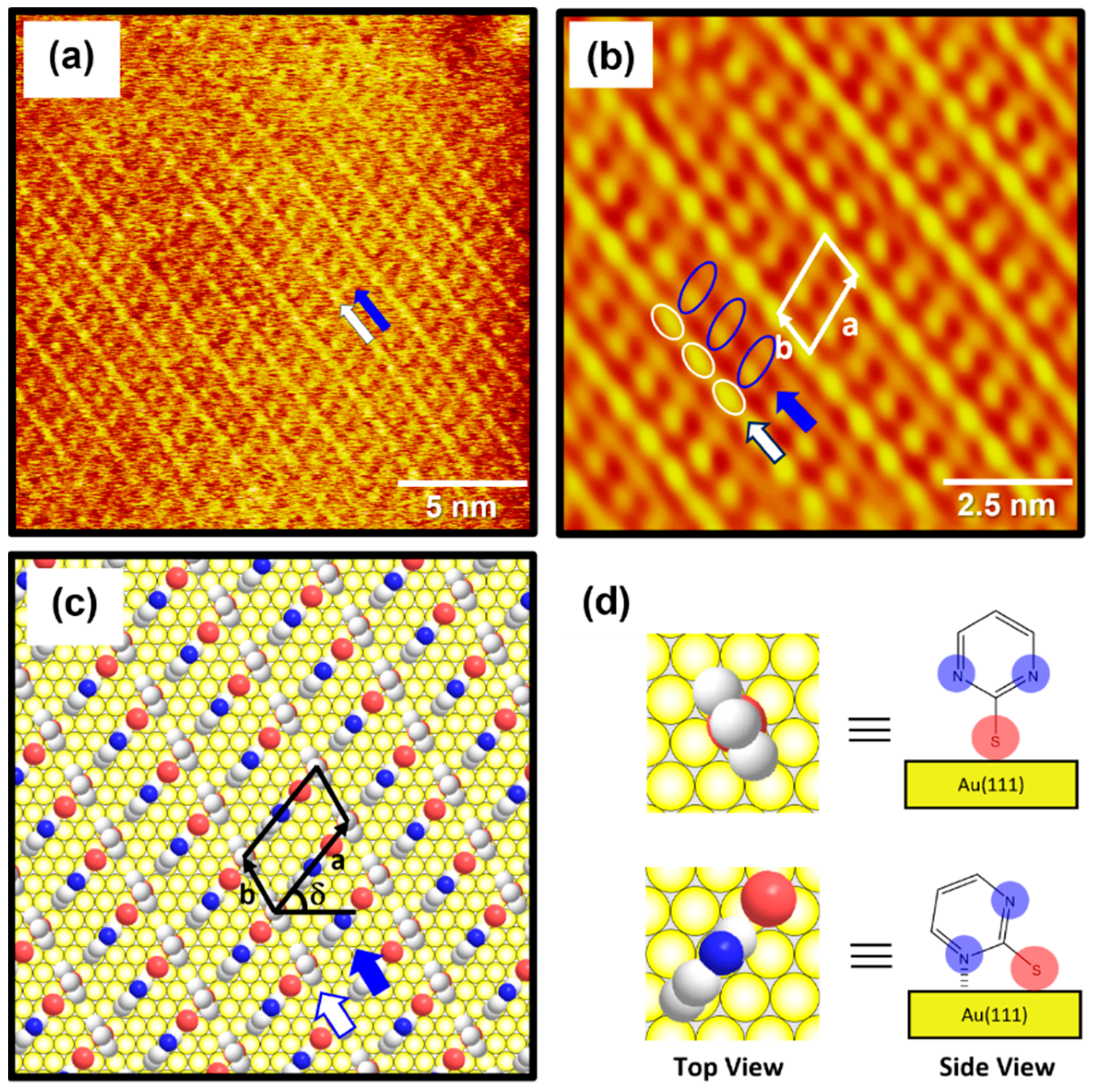
2.3. Surface Structure of Well-Ordered 2PymS SAMs on Au(111)
2.4. S 2p XPS and N 1s Peaks for 2PymS SAMs on Au(111) at Different Solution pHs
3. Experimental
3.1. Synthesis of DPymDS
3.2. Preparation of 2PymS SAMs on Au(111)
3.3. STM and XPS Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.; Hou, J.; Sotthewes, K. Probing surface properties of organic molecular layers by scanning tunneling microscopy. Adv. Colloid Interface Sci. 2023, 318, 102956. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jeong, H.; Seong, S.; Han, S.; Son, Y.J.; Tahara, H.; Hayashi, T.; Yoon, H.J.; Noh, J. Formation and superlattice of long-range and highly ordered alicyclic selenolate monolayers on Au(111) studied by scanning tunneling microscopy. Appl. Surf. Sci. 2022, 572, 151454. [Google Scholar] [CrossRef]
- Muneyasu, R.; Yamada, T.; Akai-Kasaya, M.; Kato, H.S. Self-assembly of heterogeneous bilayers stratified by Au-S and hydrogen bonds on Au(111). Phys. Chem. Chem. Phys. 2022, 24, 22222–22230. [Google Scholar] [CrossRef]
- Asyuda, A.; Das, S.; Zharnikov, M. Thermal stability of alkanethiolate and aromatic thiolate self-assembled monolayers on Au(111): An X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2021, 125, 21754–21763. [Google Scholar] [CrossRef]
- Seong, S.; Kang, H.; Kim, H.; Son, Y.J.; Jang, J.; Maeda, S.; Chikami, S.; Hayashi, T.; Yoon, H.J.; Noh, J. Effects of the substituent position on the structural order, work function change, and thermopower of dichloro-substituted benzenethiolate self-assembled monolayers on Au(111). Appl. Surf. Sci. 2024, 642, 158661. [Google Scholar] [CrossRef]
- Son, Y.J.; Kang, H.; Seong, S.; Han, S.; Lee, N.S.; Noh, J. Thermally driven structural order of oligo(ethylene glycol)-terminated alkanethiol monolayers on Au(111) prepared by vapor deposition. Molecules 2022, 27, 5377. [Google Scholar] [CrossRef]
- Son, Y.J.; Han, J.W.; Kang, H.; Seong, S.; Han, S.; Maeda, S.; Chikami, S.; Hayashi, T.; Hara, M.; Noh, J. Formation and thermal stability of ordered self-assembled monolayers by the adsorption of amide-containing alkanethiols on Au(111). Int. J. Mol. Sci. 2023, 24, 3241. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Byeon, S.E.; Yoon, H.J. N-heterocyclic carbene anchors in electronics applications. Bull. Korean Chem. Soc. 2021, 42, 712–723. [Google Scholar] [CrossRef]
- Kim, K.J.; Song, Y.; Park, S.; Oh, S.J.; Kwon, S.J. Immunosensor for human IgE detection using electrochemical redox cycling with ferrocene-mixed self-assembled monolayers modified Au electrode. Bull. Korean Chem. Soc. 2023, 44, 141–146. [Google Scholar] [CrossRef]
- Markov, A.; Wolf, N.; Yuan, X.; Mayer, D.; Maybeck, V.; Offenhäusser, A.; Wördenweber, R. Controoled engineering of oxide surface for bioelectronics applications using organic mixed monolayers. ACS Appl. Mater. Interfaces 2017, 9, 29265–29272. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, S.J.; Byeon, S.E.; He, X.; Yoon, H.J. Self-assembled monolayers as interface engineering nanomaterials in perovskite solar cells. Adv. Energy Mater. 2020, 10, 2002606. [Google Scholar] [CrossRef]
- Mutlu, A.; Can, M.; Tozlu, C. Performance improvement of organic solar cell via incorporation of donor type self-assembled interfacial monolayer. Thin Solid Films 2019, 685, 88–96. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Kang, H.; Seong, S.; Ito, E.; Isoshima, T.; Hara, M.; Yoon, H.J.; Noh, J. Comparative study of structural order, thermal desorption behavior, and work function change of self-assembled monolayers of pentafluorobenzenethiols and tetrafluorobenzenethiols on Au(111). Appl. Surf. Sci. 2021, 555, 149671. [Google Scholar] [CrossRef]
- Seong, S.; Kang, H.; Han, S.; Son, Y.J.; Jang, J.; Yoon, H.J.; Maeda, S.; Song, S.; Palai, D.; Hayashi, T.; et al. Surface structure and work function change of pentafluorobenzeneselenolate self-assembled monolayers on Au (111). Surf. Interfaces 2022, 33, 102228. [Google Scholar] [CrossRef]
- Tsvetanova, M.; Oldenkotte, V.J.S.; Bertolino, M.C.; Gao, Y.; Siekman, M.H.; Huskens, J.; Zandvliet, H.J.W.; Sottewes, K. Nanoscale work function contrast induced by decanethiol self-assembled monolayers on Au(111). Langmuir 2020, 36, 12745–12754. [Google Scholar] [CrossRef]
- Hamadani, B.H.; Corley, D.A.; Ciszek, J.W.; Tour, J.M.; Natelson, D. Controlling charge injection in organic field-effect transistors using self-assembled monolayers. Nano Lett. 2006, 6, 1303–1306. [Google Scholar] [CrossRef]
- Cho, S.J.; Kong, G.D.; Park, S.; Park, J.; Byeon, S.E.; Kim, T.; Yoon, H.J. Molecularly controlled stark effect induces significant rectification in polycyclic-aromatic-hydrocarbon-terminated N-alkanethiolates. Nano Lett. 2019, 19, 545–553. [Google Scholar] [CrossRef]
- Kong, L.; Chesneau, F.; Zhang, Z.; Staier, F.; Terfort, A.; Dowben, P.A.; Zharnikov, M. Electronic structure of aromatic monomolecular films: The effect of molecular spacers and interfacial dipoles. J. Phys. Chem. C 2011, 115, 22422–22428. [Google Scholar] [CrossRef]
- Heimel, G.; Rissner, F.; Zojer, E. Modeling the electronic properties of π-conjugated self-assembled monolayers. Adv. Mater. 2010, 22, 2494–2513. [Google Scholar] [CrossRef] [PubMed]
- Heimel, G.; Romaner, L.; Brédas, J.L.; Zojer, E. Interface energetics and level alignment at covalent metal-molecule junctions: π-conjugated thiols on gold. Phys. Rev. Lett. 2006, 96, 196806. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, L.; Chen, W.; Feng, Y.; Wee, A.T.S. Configuration-dependent interface charge transfer at a molecule-metal junction. J. Am. Chem. Soc. 2006, 128, 8003–8007. [Google Scholar] [CrossRef] [PubMed]
- Thuo, M.M.; Reus, W.F.; Nijhuis, C.A.; Barber, J.R.; Kim, C.; Schulz, M.D.; Whitesides, G.M. Odd-even effects in charge transport across self-assembled monolayers. J. Am. Chem. Soc. 2011, 133, 2962–2975. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, H. Molecular electron transport changes upon structural phase transitions in alkanethiol molecular junctions. ACS Nano 2009, 3, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S. Molecular assemblies of functional molecules on gold electrode surfaces studied by electrochemical scanning tunneling microscopy: Relationship between function and adlayer structures. Bull. Chem. Soc. Jpn. 2006, 79, 1167–1190. [Google Scholar] [CrossRef]
- Monari, S.; Ranieri, A.; Bortolotti, C.A.; Peressini, S.; Tavagnacco, C.; Borsari, M. Unfolding of cytochrome c immobilized on self-assembled monolayers. An electrochemical study. Electrochim. Acta 2011, 56, 6925–6931. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Mizutani, F.; Yoshimoto, S.; Taniguchi, I. Voltammetric and in situ STM studies on self-assembled monolayers of 4-mercaptopyridine, 2-mercaptopyridine and thiophenol on Au(111) electrodes. Electrochim. Acta 2000, 45, 2861–2867. [Google Scholar] [CrossRef]
- Pang, Y.S.; Hwang, H.J.; Kim, M.S. Adsorption of 2-mercaptopyridine and 2-mercaptopyrimidine on a silver colloidal surface investigated by Raman spectroscopy. J. Mol. Stru. 1998, 441, 63–76. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshimoto, S.; Sunatsuki, Y.; Nishiyanma, K. Potential and pH dependencies of adsorbed species of 2-, 4-pyridinethiol and 2-pyrimidinethiol on Au(111) electrode. Electrochemistry 1999, 67, 1197–1199. [Google Scholar] [CrossRef]
- Zhang, H.L.; Evans, S.D.; Henderson, J.R.; Miles, R.E.; Shen, T. Spectroscopic characterization of gold nanoparticles passivated by mercaptopyridine and mercaptopyrimidine derivatives. J. Phys. Chem. B 2003, 107, 6087–6095. [Google Scholar] [CrossRef]
- Noh, J.; Ito, E.; Hara, M. Self-assembled monolayers of benzenethiol and benzenemethanethiol on Au(111): Influence of an alkyl spacer on the structure and thermal desorption behavior. J. Colloid Interface Sci. 2010, 342, 513–517. [Google Scholar] [CrossRef]
- Yang, G.; Liu, G.Y. New insights for self-assembled monolayers of organothiols on Au(111) revealed by scanning tunneling microscopy. J. Phys. Chem. B 2003, 107, 8746–8759. [Google Scholar] [CrossRef]
- Kang, H.; Park, T.; Choi, I.; Lee, Y.; Ito, E.; Hara, M.; Noh, J. Formation of large ordered domains in benzenethiol self-assembled monolayers on Au(111) observed by scanning tunneling microscopy. Ultramicroscopy 2009, 109, 1011–1014. [Google Scholar] [CrossRef]
- Davis, J.J.; Hill, H.A.O.; Yamada, R.; Naohara, H.; Uosaki, K. Scanning tunnelling microscopy study of the self assembly of 2-mercaptopyrimidine and 4,6-dimethyl-2-mercaptopyrimidine on on Au(111). J. Chem. Soc. Faraday Trans. 1998, 94, 1315–1319. [Google Scholar] [CrossRef]
- Pinheiro, L.S.; Temperini, M.L.A. 2-Mercaptopyrimidine as a suitable matrix to trap arenes on Au(111): STM probing of the modified surface. Curr. Appl. Phys. 2002, 2, 145–153. [Google Scholar] [CrossRef]
- Freeman, F.; Po, H.N. Dimers of and tautomerism between 2-pyrimidinethiol and 2(1H)-pyrimidinethione: A density functional theory (DFT) study. J. Phys. Chem. A 2006, 110, 7904–7912. [Google Scholar] [CrossRef]
- Bain, C.D.; Biebuyck, H.A.; Whitesides, G.M. Comparison of self-assembled monolayers on gold: Coadsorption of thiols and disulfides. Langmuir 1989, 3, 3891–3897. [Google Scholar] [CrossRef]
- Szafranski, C.A.; Tanner, W.; Laibinis, P.E.; Garrell, R.L. Surface-enhanced Raman spectroscopy of aromatic thiols and disulfides on gold electrodes. Langmuir 1998, 14, 3570–3579. [Google Scholar] [CrossRef]
- Schreiber, F. Structure and growth of self-assembling monolayers. Prog. Surf. Sci. 2000, 65, 151–256. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chang, T.C.; Lee, Y.L. Adsorption behavior of 11-mercapto-1-undecanol on Au(111) electrode in an electrochemical system. J. Phys. Chem. C 2007, 111, 4014–4020. [Google Scholar] [CrossRef]
- Kang, H.; Ito, E.; Hayashi, T.; Hara, M.; Noh, J. Effect of solution concentration on the formation of ordered domains in pentachlorobenzenethiol self-assembled monolayers on Au(111). J. Nanosci. Nanotechol. 2016, 16, 6360–6363. [Google Scholar] [CrossRef]
- Azzam, W.; Bashir, A.; Ulrich Biedermann, P.; Rohwerder, M. Formation of highly ordered and orientated gold islands: Effect of immersion time on the molecular adlayer structure of pentafluorobenzenethiols (PFBT) SAMs on Au(111). Langmuir 2012, 28, 10192–10208. [Google Scholar] [CrossRef]
- Nishiyama, K.; Tsuchiyama, M.; Kubo, A.; Seriu, H.; Miyazaki, S.; Yoshimoto, S.; Taniguchi, I. Conformational change in 4-pyridineethanethiolate self-assembled monolayers on Au(111) driven by protonation/deprotonation in electrolyte solutions. Phys. Chem. Chem. Phys. 2008, 10, 6935–6939. [Google Scholar] [CrossRef]
- Wattanavichean, N.; Casey, E.; Nochols, R.J.; Arnolds, H. Discrimination between hydrogen bonding and protonation in the spectra of a surface-enhanced Raman sensor. Phys. Chem. Chem. Phys. 2018, 20, 866–871. [Google Scholar] [CrossRef]
- Cui, B.; Chen, T.; Wang, D.; Wan, L.J. In situ STM evidence for the adsorption geometry of three N-heteroaromatic thiols on Au(111). Langmuir 2011, 27, 7614–7619. [Google Scholar] [CrossRef]
- Ohms, V.H.; Guth, H.; Kutoglu, A.; Scheringer, C. 2-Thiopyridone: X-ray and neutron diffraction study. Acta Crystallogr. Sect. B 1982, 38, 831. [Google Scholar] [CrossRef]
- Ishida, T.; Hara, M.; Kojima, I.; Tsuneda, S.; Nishida, N.; Sasabe, H.; Knoll, W. High-resolution X-ray photoelectron spectroscopy measurements of octadecanethiol self-assembled monolayers on Au(111). Langmuir 1998, 14, 2092–2096. [Google Scholar] [CrossRef]
- Noh, J.; Jang, S.; Lee, D.; Shin, S.; Ko, Y.J.; Ito, E.; Joo, S.W. Abnormal adsorption behavior of dimethyl disulfide on gold surfaces. Curr. Appl. Phys. 2007, 7, 605–610. [Google Scholar] [CrossRef]
- Cometto, F.P.; Patrito, E.M.; Olivara, P.P.; Zampieri, G.; Ascolani, H. Electrochemical, high-resolution photoemission spectroscopy and vdW-DFT study of the thermal stability of benzenethiol and benzeneselenol monolayers on Au(111). Langmuir 2012, 28, 13624–13635. [Google Scholar] [CrossRef]
- Castner, D.G.; Hinds, K.; Grainger, D.W. X-ray photoelectron spectroscopy sulfur 2p study of organic thiol and disulfide binding interactions with gold surfaces. Langmuir 1996, 12, 5083–5086. [Google Scholar] [CrossRef]
- Mamun, A.H.A.; Hahn, J.R. Effects of Solvent on the Formation of Octanethiol Self-Assembled Monolayers on Au(111) at High Temperatures in a Closed Vessel: A Scanning Tunneling Microscopy and X-ray Photoelectron Spectroscopy Study. J. Phys. Chem. C 2012, 116, 22441–22448. [Google Scholar] [CrossRef]
- Herrera, S.H.; Tasca, F.; Williams, F.J.; Calvo, E.J. Surface structure of 4-mercaptopyridine on Au(111): A new dense phase. Langmuir 2017, 33, 9565–9572. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Dutta, B.; Ghosh, M.; Chowdhury, J. Adsorption of 4-mercaptopyridine with gold nanoparticles embedded in the Langmuir-Blodgett film matrix of stearic acid: SERS, XPS studies aided by Born-Oppenheimer on the fly dynamics, time-resolved wavelet transform theory, DFT. ACS Omega 2022, 7, 27818–27830. [Google Scholar] [CrossRef] [PubMed]
- Kirihara, M.; Asai, Y.; Ogawa, S.; Noguchi, T.; Hatano, A.; Hirai, Y. Mild and environmentally benign oxidation of thiols to disulfides. Synthesis 2007, 21, 3286–3289. [Google Scholar] [CrossRef]





| pH Condition | S 2p Species | Peak (eV) a | S 2p/Au 4f b | S1 + S2/Au 4f b |
|---|---|---|---|---|
| pH = 2 | S1 | 161.29 | 0.00029 | 0.00225 |
| S2 | 162.12 | 0.00196 | ||
| S3 | 163.58 | 0.00050 | ||
| pH = 7 | S1 | 161.24 | 0.00044 | 0.00215 |
| S2 | 162.07 | 0.00171 | ||
| S3 | 163.38 | 0.00049 | ||
| pH = 12 | S1 | 160.95 | 0.00057 | 0.00289 |
| S2 | 162.04 | 0.00232 | ||
| S3 | 163.66 | 0.00049 |
| pH Condition | N 1s Species | Peak (eV) | N 1s/Au 4f a | (N1 + N2)/Au 4f a |
|---|---|---|---|---|
| pH = 2 | N1 | 398.74 | 0.00370 | 0.00437 |
| N2 | 399.74 | 0.00067 | ||
| N3 | 400.51 | 000051 | ||
| N4 | 401.52 | 0.00024 | ||
| pH = 7 | N1 | 398.84 | 0.00405 | 0.00468 |
| N2 | 399.85 | 0.00063 | ||
| N3 | 400.56 | 0.00091 | ||
| N4 | 401.57 | 0.00044 | ||
| pH = 12 | N1 | 398.83 | 0.00420 | 0.00567 |
| N2 | 39975 | 0.00147 | ||
| N3 | 400.55 | 0.00016 | ||
| N4 | 401.38 | 0.00020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.; Seong, S.; Kim, H.; Oh, H.S.; Lee, J.H.; Kim, H.; Kim, Y.O.; Maeda, S.; Chikami, S.; Hayashi, T.; et al. Molecular Self-Assembly and Adsorption Structure of 2,2′-Dipyrimidyl Disulfides on Au(111) Surfaces. Molecules 2024, 29, 846. https://doi.org/10.3390/molecules29040846
Seo D, Seong S, Kim H, Oh HS, Lee JH, Kim H, Kim YO, Maeda S, Chikami S, Hayashi T, et al. Molecular Self-Assembly and Adsorption Structure of 2,2′-Dipyrimidyl Disulfides on Au(111) Surfaces. Molecules. 2024; 29(4):846. https://doi.org/10.3390/molecules29040846
Chicago/Turabian StyleSeo, Dongjin, Sicheon Seong, Haeri Kim, Hyun Su Oh, Jun Hyeong Lee, Hongki Kim, Yeon O Kim, Shoichi Maeda, Shunta Chikami, Tomohiro Hayashi, and et al. 2024. "Molecular Self-Assembly and Adsorption Structure of 2,2′-Dipyrimidyl Disulfides on Au(111) Surfaces" Molecules 29, no. 4: 846. https://doi.org/10.3390/molecules29040846






