Orientation Growth of N-Doped and Iron-Based Metal–Organic Framework and Its Application for Removal of Cr(VI) in Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of NH2-Functionalized Nano-Sized Magnetic MOFs
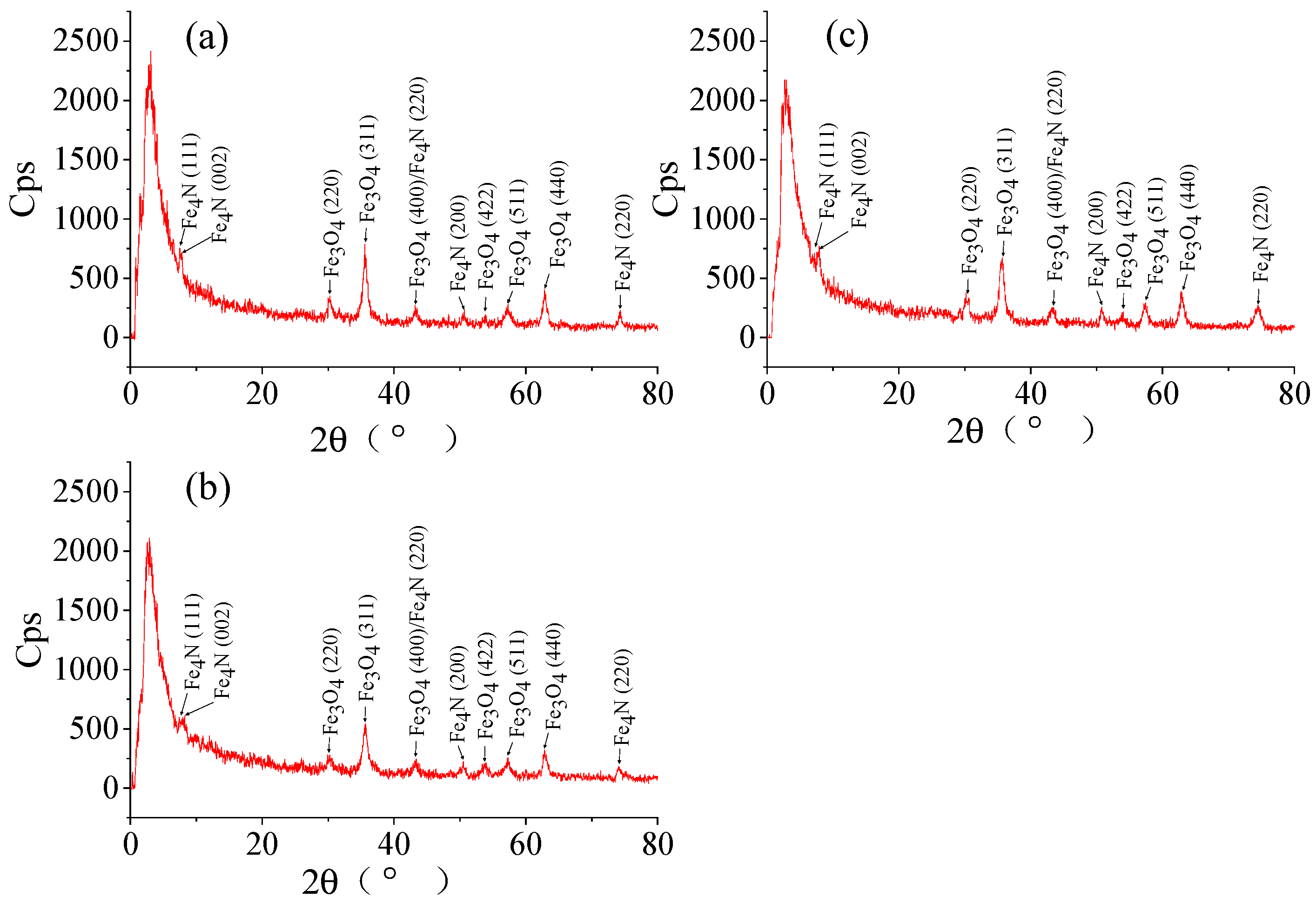
2.1.1. SEM and XRD Analyses of NH2-Functionalized Nano-Sized Magnetic MOFs
2.1.2. VSM Analysis of NH2-Functionalized Nano-Sized Magnetic MOFs
2.1.3. XPS Analysis of NH2-Functionalized Nano-Sized Magnetic MOFs
2.2. Effect of pH Value on the Adsorption Efficiencies
2.3. Effect of Initial Concentration of Cr(VI) on the Adsorption Efficiencies
2.4. Effect of Usage Amount of Ammonium Hydroxide
2.5. Kinetic Studies
2.6. Adsorption Properties Comparison
3. Experimental
3.1. Materials and Physical Measurements
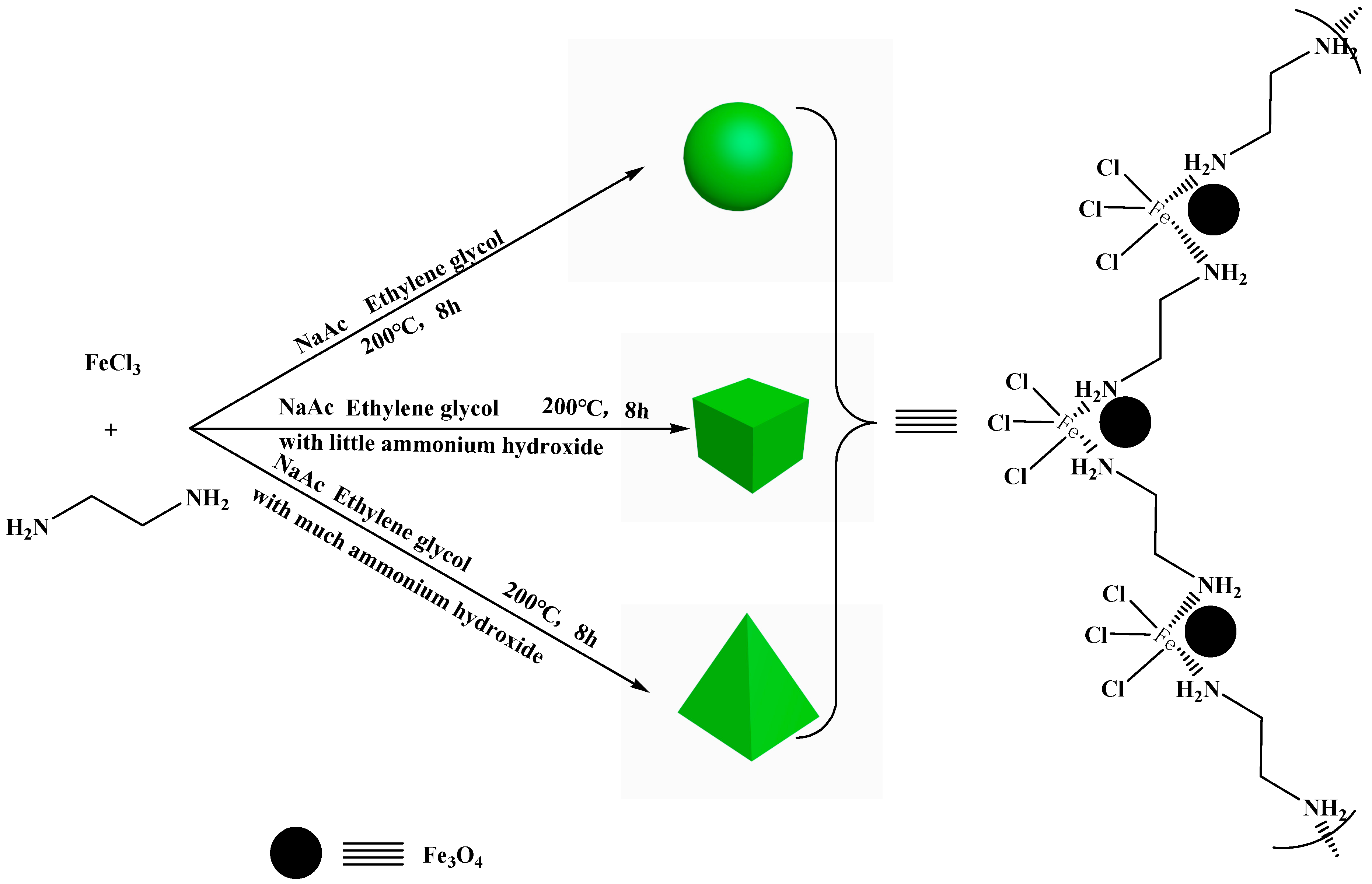
3.2. Synthesis of NH2-Functionalized Nano-Sized Magnetic MOFs
3.3. Adsorption Experiments
3.4. Analysis of Adsorption Data
3.4.1. Adsorption Model
3.4.2. Kinetic Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Lingamdinne, L.P.; Yang, J.K.; Koduru, J.R.; Chang, Y.Y.; Naushad, M. Biopolymer mixture-entrapped modified graphene oxide for sustainable treatment of heavy metal contaminated real surface water. J. Water Process Eng. 2022, 46, 102631. [Google Scholar] [CrossRef]
- Li, Y.F.; Wen, J.; Xue, Z.Z.; Yin, X.Y.; Yuan, L.; Yang, C.L. Removal of Cr(VI) by polyaniline embedded polyvinyl alcohol/sodium alginate beads-Extension from water treatment to soil remediation. J. Hazard Mater. 2022, 426, 127809. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.W.; Siddique, M.B.A.; Shabbir, Z.; Ullah, H.; Riaz, L.; Nisa, W.-U.; Shafeequr, R.; Shah, A.A. Biotreatment potential of co-contaminants hexavalent chromium and polychlorinated biphenyls in industrial wastewater: Individual and simultaneous prospects. Sci. Total Environ. 2021, 779, 146345. [Google Scholar] [CrossRef] [PubMed]
- Mon, P.P.; Cho, P.P.; Chanadana, L.; Ashok Kumar, K.V.; Dobhal, S.; Shashidhar, T.; Madras, G.; Subrahmanyam, C. Bio-waste assisted phase transformation of Fe3O4/carbon to nZVI/graphene composites and its application in reductive elimination of Cr(VI) removal from aquifer. Sep. Purif. Technol. 2023, 306, 122632. [Google Scholar]
- Bao, S.Y.; Yang, W.W.; Wang, Y.J.; Yu, Y.S.; Sun, Y.Y. Highly efficient and ultrafast removal of Cr(VI) in aqueous solution to ppb level by poly(allylamine hydrochloride) covalently cross-linked amino-modified graphene oxide. J. Hazard. Mater. 2021, 409, 124470. [Google Scholar] [CrossRef]
- Herath, A.; Salehi, M.; Jansone-Popova, S. Production of polyacrylonitrile/ionic covalent organic framework hybrid nanofibers for effective removal of chromium (VI) from water. J. Hazard. Mater. 2022, 427, 128167. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yan, R.X.; Liu, M.; Xu, J.Q.; Hagio, T.; Ichino, R.; Li, L.; Cao, X.D. Simultaneous reduction and sequestration of hexavalent chromium by magnetic beta-Cyclodextrin stabilized Fe3S4. J. Hazard. Mater. 2022, 431, 128592. [Google Scholar] [CrossRef]
- Yin, Z.B.; Xu, S.; Liu, S.; Xu, S.Y.; Li, J.H.; Zhang, Y.C. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Fei, H.H.; Oliver, S.R.J. Copper hydroxide ethanedisulfonate: A cationic inorganic layered material for high-capacity anion exchange. Angew. Chem. Int. Ed. 2011, 50, 9066–9070. [Google Scholar] [CrossRef]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Heterogeneous photocatalytic reduction of chromium(VI) over TiO2 particles in the presence of oxalate: Involvement of Cr(V) species. Environ. Sci. Technol. 2004, 38, 1589–1594. [Google Scholar] [CrossRef]
- Desai, A.V.; Manna, B.; Karmakar, A.; Sahu, A.; Ghosh, S.K. A water-stable cationic metal-organic framework as a dual adsorbent of oxoanion pollutants. Angew. Chem. Int. Ed. 2016, 55, 7811–7815. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Tech. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Korak, J.A.; Huggins, R.; Arias-Paic, M. Regeneration of pilot-scale ion exchange columns for hexavalent chromium removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B Environ. 2017, 214, 126–136. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Pang, H.W.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.R.; Wang, X.K. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.A.; Taleb, M.A.; Seliem, M.K. A recyclable multifunctional graphene oxide/SiO2@polyaniline microspheres composite for Cu(II) and Cr(VI) decontamination from wastewater. J. Clean. Prod. 2020, 268, 122290. [Google Scholar] [CrossRef]
- Vinayagam, R.; Dave, N.; Varadavenkatesan, T.; Rajamohan, N.; Sillanpaa, M.; Nadda, A.K.; Govarthanan, M.; Selvaraj, R. Artificial neural network and statistical modelling of biosorptive removal of hexavalent chromium using macroalgal spent biomass. Chemosphere 2022, 296, 133965. [Google Scholar] [CrossRef]
- Paltrinieri, L.; Wang, M.; Sachdeva, S.; Besseling, N.A.; Sudhölter, E.J.; De Smet, L.C. Fe3O4 nanoparticles coated with a guanidinium-functionalized polyelectrolyte extend the pH range for phosphate binding. J. Mater. Chem. A 2017, 5, 18476–18485. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, F.; He, J.; Xu, H.; Cui, F.; Wang, W. Robust phosphate capture over inorganic adsorbents derived from lanthanum metal organic frameworks. Chem. Eng. J. 2017, 326, 1086–1094. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Shi, R.; Zhang, W.; Yang, X.; Shi, W.; Cui, F. High speed water purification and efficient phosphate rejection by active nanofibrous membrane for microbial contamination and regrowth control. Chem. Eng. J. 2018, 337, 428–435. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms that control the adsorption–desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Adsorption of phosphate from seawater on calcined MgMn-layered double hydroxides. J. Colloid Interface Sci. 2005, 290, 45–51. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Zeb, S.; Ali, N.; Ali, Z.; Bilal, M.; Adalat, B.; Hussain, S.; Gul, S.; Ali, F.; Ahmad, R.; Iqbal, H.M. Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. J. Water Process Eng. 2020, 38, 101675. [Google Scholar] [CrossRef]
- Meng, S.; Yu, S.; Tang, F.; Hu, X.; Lu, J.; Fei, X.; Zhu, M. Fiber engineering of silica-based aerogels with surface specificity and regenerability for continuous removal of dye pollutants from wastewaters. Microporous Mesoporous Mater. 2021, 314, 110874. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.; Wu, Z.; Xu, F.; Yang, D.; He, Q.; Li, G.; Chen, Y. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresour. Technol. 2019, 289, 121600. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, L.; Chang, N.; Liu, J.; Duan, C.; Zhou, Q.; Li, X.; Wang, X. Removal of phosphate from water by activated carbon fiber loaded with lanthanum oxide. J. Hazard. Mater. 2011, 190, 848–855. [Google Scholar] [CrossRef]
- Asif, M.B.; Li, C.; Ren, B.; Maqbool, T.; Zhang, X.; Zhang, Z. Elucidating the impacts of intermittent in-situ ozonation in a ceramic membrane bioreactor: Micropollutant removal, microbial community evolution and fouling mechanisms. J. Hazard. Mater. 2021, 402, 123730. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-based sustainable nanomaterials for water treatment: State-of-art and future perspectives. Chemosphere 2021, 263, 128005. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of organic water pollutants by clays and clay minerals composites: A comprehensive review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B.; Hummadi, E. Review on recent progress in chitosan/chitincarbonaceous material composites for the adsorption of water pollutants. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of chitin and chitosan based biomaterials for the adsorptive removal of textile dyes from water-A comprehensive review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef]
- Saheed, I.O.; Da Oh, W.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Liu, T.; Chang, B.; Wu, K. The performance of phosphate removal using aluminiummanganese bimetal oxide coated zeolite: Batch and dynamic adsorption studies. Desalination Water Treat. 2016, 57, 4220–4233. [Google Scholar] [CrossRef]
- Thakur, K.; Kandasubramanian, B. Graphene and graphene oxide-based composites for removal of organic pollutants: A review. J. Chem. Eng. Data 2019, 64, 833–867. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B. Adsorption and co-adsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem. Eng. J. 2015, 281, 379–388. [Google Scholar] [CrossRef]
- Yao, N.; Li, C.; Yu, J.; Xu, Q.; Wei, S.; Tian, Z.; Yang, Z.; Yang, W.; Shen, J. Insight into adsorption of combined antibiotic-heavy metal contaminants on graphene oxide in water. Sep. Purif. Technol. 2020, 236, 116278. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.I.; Milanović, M.; Stijepović, I.; Almásy, L. Comparison of structure and adsorption properties of mesoporous silica functionalized with aminopropyl groups by the co-condensation and the post grafting methods. Materials 2021, 14, 628. [Google Scholar] [CrossRef]
- Suručić, L.; Janjić, G.; Marković, B.; Tadić, T.; Vuković, Z.; Nastasović, A.; Onjia, A. Speciation of hexavalent chromium in aqueous solutions using a magnetic silica-coated amino-modified glycidyl methacrylate polymer nanocomposite. Materials 2023, 16, 2233. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; de Fernandez-de Luis, R.; Salazar, H.; Gonçalves, B.F.; King, S.; Almásy, L.; Kriechbaum, M.; Laza, J.M.; Vilas-Vilela, J.L.; Martins, P.M.; et al. On the multiscale structure and morphology of PVDF-HFP@MOF membranes in the scope of water remediation applications. Adv. Mater. Interfaces 2023, 10, 2300424. [Google Scholar] [CrossRef]
- El-Mehalmey, W.A.; Ibrahim, A.H.; Abugable, A.A.; Hassan, M.H.; Haikal, R.R.; Karakalos, S.G.; Zaki, O.; Alkordi, M.H. Metal-organic framework@silica as a stationary phase sorbent for rapid and cost-effective removal of hexavalent chromium. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 2742–2751. [Google Scholar] [CrossRef]
- Luo, M.B.; Xiong, Y.Y.; Wu, H.Q.; Feng, X.F.; Li, J.Q.; Luo, F. The MOF technique: A significant synergic effect enables high performance chromate removal. Angew. Chem. Int. Ed. 2017, 56, 16376. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.C.; Chen, B.L.; Qian, G.D. A porous Zr-cluster-based cationic metal organic framework for highly efficient Cr2O72− removal from water. Chem. Commun. 2015, 51, 14732–14734. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, B.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K.; Cao, X.; Liu, H.; Liu, H. Microbial vanadium (V) reduction in groundwater with different soils from vanadium ore mining areas. Chemosphere 2018, 202, 272–279. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.Z.; Kong, X.J.; Yu, J.; Lv, X.L.; Wu, Y.; Guo, Z.J.; Li, J.R. Zr(IV)-Based Metal-Organic Framework with T-Shaped Ligand: Unique Structure, High Stability, Selective Detection, and Rapid Adsorption of Cr2O72− in Water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zheng, H.Q.; Zheng, H.Y.; Lin, L.P.; Xin, Q.; Cao, R. Efficient capture and effective sensing of Cr2O72− from water using a zirconium metal-organic framework. Inorg. Chem. 2017, 56, 14178–14188. [Google Scholar] [CrossRef]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Sun, L.; Shi, H.; Shi, C.; Liang, Z.; Li, J. A zwitterionic ligand-based cationic metal-organic framework for rapidly selective dye capture and highly efficient Cr2O72− removal. Chem. Eur. J. 2018, 24, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Han, C.S.; Robins, J.C.; Oliver, S.R.J. A cationic metal-organic solid solution based on Co(II) and Zn(II) for chromate trapping. Chem. Mater. 2013, 25, 647–652. [Google Scholar] [CrossRef]
- Shao, Z.; Huang, C.; Wu, Q.; Zhao, Y.; Xu, W.; Liu, Y.; Dang, J.; Hou, H. Ion exchange collaborating coordination substitution: More efficient Cr(VI) removal performance of a water-stable Cu(II)-MOF material. J. Hazard. Mater. 2019, 378, 120719. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, G.H.; Lo, I.M.C. Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: Performance and mechanisms. J. Environ. Eng. 2006, 132, 709–715. [Google Scholar] [CrossRef]
- Zhao, Y.-G.; Shen, H.-Y.; Pan, S.-D.; Hu, M.-Q. Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J. Hazard. Mater. 2010, 182, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.R.; Zhai, X.J.; Wang, S.; Xu, H.; Wang, R.; Zang, S.Q. 3D-ordered macroporous N-doped carbon encapsulating Fe-N alloy derived from a single-source metal organic framework for superior oxygen reduction reaction. Chin. J. Catal. 2021, 42, 490–500. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, R.; Liu, L.; Zhang, Y.; Hu, P. Structure and properties of nanoscale zero-valent iron supported on multimorphologies kaolinite and its enhanced removal of Cr(VI) from water. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132069. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, F.; Liu, X.; Li, M. Removal of Cr(VI) by glutaraldehyde-crosslinked chitosan encapsulating microscale zero-valent iron: Synthesis, mechanism, and longevity. J. Environ. Sci. 2023, 142, 115–128. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhang, Y.; Zhao, K.; Feng, X. Selective removal of Cr(VI) by tannic acid and polyethyleneimine modified zero-valent iron particles with air stability. J. Hazard. Mater. 2023, 458, 132018. [Google Scholar] [CrossRef]
- Ma, J.; Jia, N.; Jin, H.; Yao, S.; Zhang, K.; Kai, Y.; Wu, W.; Wen, Y. Chitosan induced synthesis of few-layer MoS2/Fe-doped biochar and its dual applications in Cr(VI) removal. Sep. Purif. Technol. 2023, 317, 123880. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, S.; Fu, L.; Zhang, L. The one-step synthesis of a novel metal–organic frameworks for efficient and selective removal of Cr(VI) and Pb(II) from wastewater: Kinetics, thermodynamics and adsorption mechanisms. J. Colloid Interface Sci. 2023, 640, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, W.; Gao, H.; Li, C.; Liang, W.; Nie, Y.; Shen, C.; Ai, S. A novel aminated lignin/geopolymer supported with Fe nanoparticles for removing Cr(VI) and naphthalene: Intermediates promoting the reduction of Cr(VI). Sci. Total Environ. 2023, 866, 161379. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, W.; Huang, B.; Chen, Y.; Ren, X.; Shen, Y.; Zhou, Y.; Cheng, R.; Zhang, J.; Qiu, M.; et al. Efficient removal of Cr(VI) by the modified biochar with chitosan schiff base and MnFe2O4 nanoparticles: Adsorption and mechanism analysis. J. Environ. Chem. Eng. 2023, 11, 109432. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, H.; Wang, Z.; Li, D.; Shen, X.; Xu, X.; Li, K.; Xiang, Q.; Wu, Y.; Chen, Q. Preparation of N-doped cellulose-based hydrothermal carbon using a two-step hydrothermal induction assembly method for the efficient removal of Cr(VI) from wastewater. Environ. Res. 2023, 219, 115015. [Google Scholar] [CrossRef]






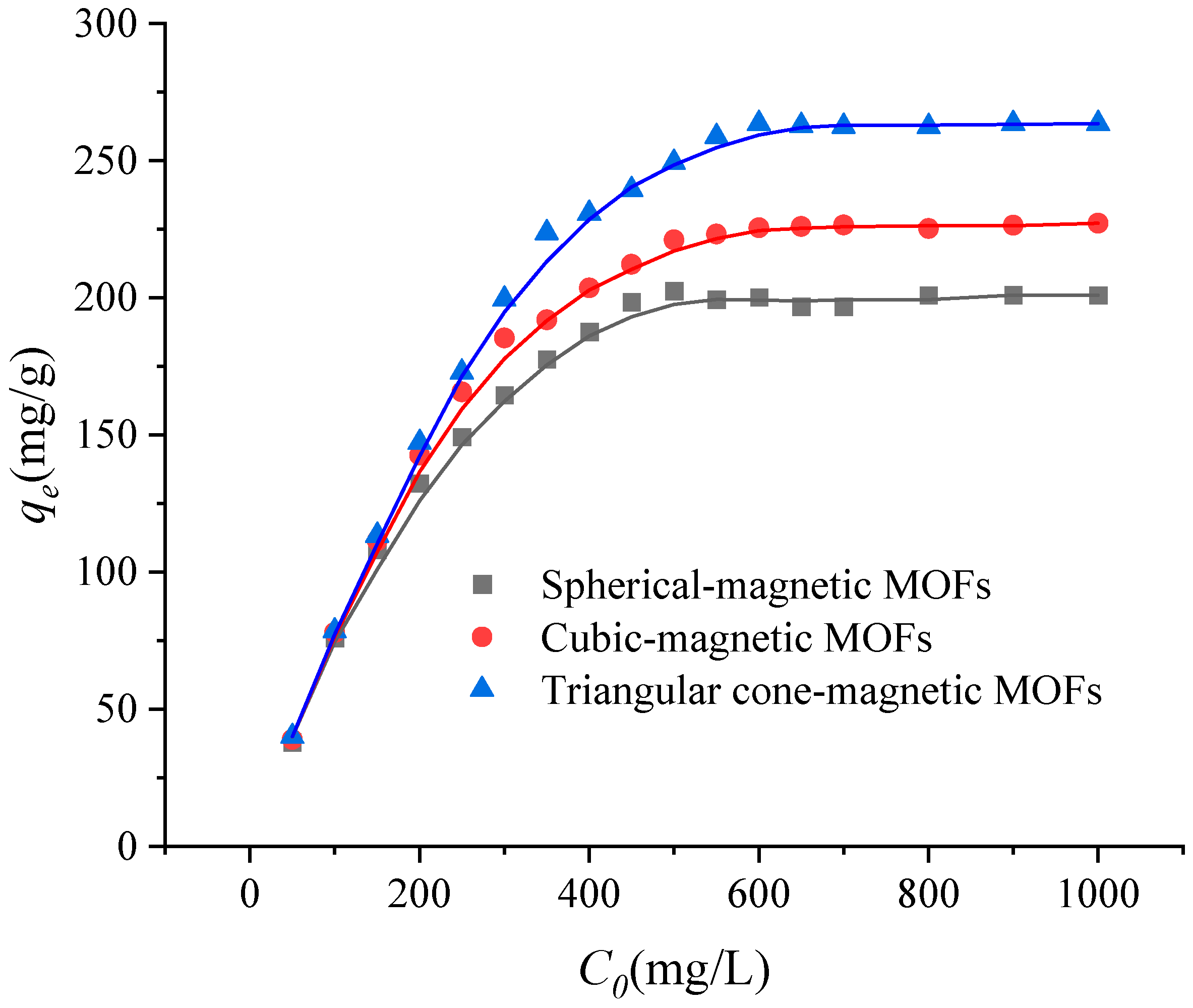


| Magnetic MOFs | Langmuir Isotherms | Langmuir Constants | ||
|---|---|---|---|---|
| K (L/mg) | R2 | qm (mg/g) | ||
| Spherical-magnetic MOFs | Ce/qe = 0.0049Ce + 0.0652 | 0.0752 | 0.9992 | 204.08 |
| Cubic-magnetic MOFs | Ce/qe = 0.0043Ce + 0.0531 | 0.0810 | 0.9994 | 232.56 |
| Triangular cone-magnetic MOFs | Ce/qe = 0.0037Ce + 0.0443 | 0.0835 | 0.9994 | 270.27 |
| Magnetic MOFs | Pseudo-Second-Order Rate Equations | k2 [(g/mg)/min] | qt (mg/g) | qe.c (mg/g) | k2qe.c2 [(mg/g)/min] | R2 |
|---|---|---|---|---|---|---|
| Spherical-magnetic MOFs | t/qt = 0.0264t + 0.0042 | 0.1660 | 37.85 | 37.87 | 238.1 | 1 |
| Cubic-magnetic MOFs | t/qt = 0.0258t + 0.0040 | 0.1664 | 38.79 | 38.76 | 250.0 | 1 |
| Triangular cone-magnetic MOFs | t/qt = 0.0251t + 0.0038 | 0.1658 | 39.96 | 39.84 | 263.2 | 1 |
| Adsorbents | Equilibrium Time (min) | pH | qm (mg/g) | Ref. |
|---|---|---|---|---|
| water-stable Cu(II)-MOF material | 180 | 6.0 | 190 | [50] |
| EDA-MPs | 5~60 | 2.5 | 32.15~61.35 | [55] |
| nZVI/Kaol | 10 | <4.0 | 15.98 | [57] |
| mZVI/GCS | 288 h | 2.5 | 243.63 | [58] |
| Fe-TA-PEI-10 | 120 | 3.0 | 161.6 | [59] |
| Mo-Fe0.5-CS | 30 | - | 180 | [60] |
| MOF-DFSA | 120 | 4.0 | 188.12 | [61] |
| Fe@N-L-GM | 300 | 2.0 | 65.83 | [62] |
| CsSB/MnFe2O4@BC | 360 | 2.0 | 125.34 | [63] |
| N–CHC | 240 | 2.0 | 151.05 | [64] |
| Spherical-magnetic MOFs | 30 | 2.0 | 204.08 | This work |
| Cubic-magnetic MOFs | 30 | 2.0 | 232.56 | This work |
| Triangular cone-magnetic MOFs | 30 | 2.0 | 270.27 | This work |
| Spectrometer Parameter | |
| (a) | |
| Wavelength | 357.9 nm |
| Slit width | 0.2 nm |
| Lamp current | 8 mA |
| Burnet height | 9 mm |
| Background correction | Deuterium lamp |
| Parameters | |
| (b) | |
| Linearity range | 50–1250 μg·L−1 |
| Detection limit | 20 μg·L−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lei, C.; Zhao, Y.-G.; Ye, M.-L.; Yang, K. Orientation Growth of N-Doped and Iron-Based Metal–Organic Framework and Its Application for Removal of Cr(VI) in Wastewater. Molecules 2024, 29, 1007. https://doi.org/10.3390/molecules29051007
Chen Y, Lei C, Zhao Y-G, Ye M-L, Yang K. Orientation Growth of N-Doped and Iron-Based Metal–Organic Framework and Its Application for Removal of Cr(VI) in Wastewater. Molecules. 2024; 29(5):1007. https://doi.org/10.3390/molecules29051007
Chicago/Turabian StyleChen, Yan, Chao Lei, Yong-Gang Zhao, Ming-Li Ye, and Kun Yang. 2024. "Orientation Growth of N-Doped and Iron-Based Metal–Organic Framework and Its Application for Removal of Cr(VI) in Wastewater" Molecules 29, no. 5: 1007. https://doi.org/10.3390/molecules29051007






