Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. Quercetin Decreased Viability and Changed Cellular Morphology in Melanoma Cells
2.2. Quercetin Induced Apoptosis in Melanoma Cells
2.3. Quercetin Regulated Cell-Cycle Arrest and Apoptosis-Related Protein in Melanoma Cells
2.4. Quercetin Changed MMP and Induced Release of Cytochrome C from Intermembrane Space in Melanoma Cells
2.5. Quercetin Regulated Expression of Proliferation-Related Molecules in Melanoma Cells
2.6. Quercetin Inhibited Malignant Melanoma Cell Migration
2.7. Quercetin Reduced Ganglioside GD3 Expression in Melanoma Cells
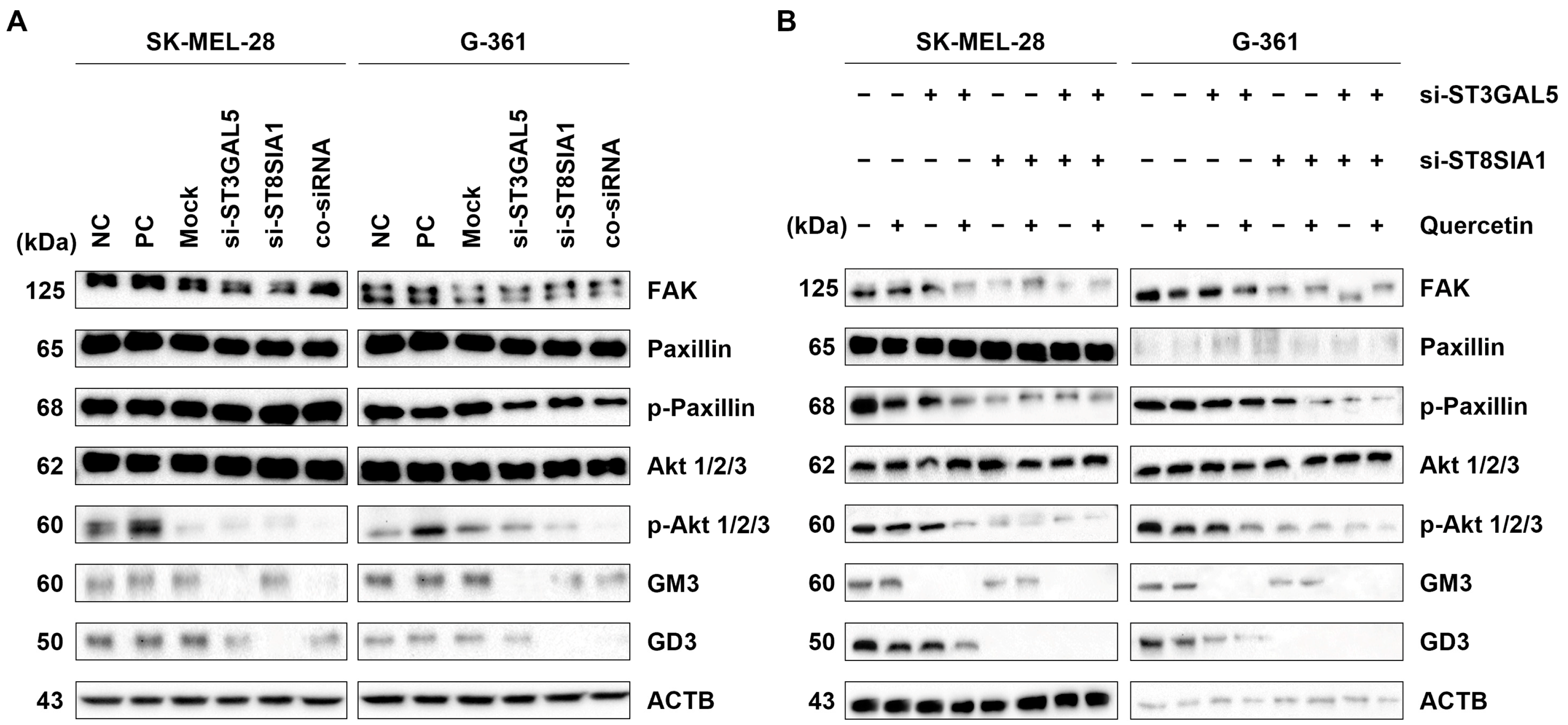
2.8. Reduced Ganglioside Expression Levels by ST8SIA1 and/or ST3GAL5 Knockdown in Melanoma Cells
2.9. Quercetin and Knockdown Ganglioside Decreased Gene Expression of FAK, Paxillin, and Akt Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagent and Antibodies
4.2. Cell Culture
4.3. MTT Cell Viability Assay
4.4. Nuclear Staining with DAPI
4.5. Cell-Cycle Analysis
4.6. Annexin V & Dead Cell Assay
4.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.8. Mitochondrial Membrane Potential (MMP) Assay
4.9. Isolation of Mitochondrial and Cytosolic Protein Fractionation
4.10. Immunoblotting Analysis
4.11. Immunocytochemistry Assay
4.12. Wound Healing Assay
4.13. Ganglioside Extraction and Purification
4.14. High-Performance Thin-Layer Chromatography (HPTLC)
4.15. Knockdown of Ganglioside ST3GAL5 and ST8SIA1 Gene by Small Interfering RNA (siRNA)
4.16. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D.L.; Cummins, J.M.; Pantle, H.; Silverman, M.A.; Leonard, A.L.; Chanmugam, A. Cutaneous malignant melanoma. Mayo Clin. Proc. 2016, 81, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 36, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C.; Centers for Disease Control and Prevention. Vital signs, melanoma incidence and mortality trends and projections—United Staes, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Bombelli, F.B.; Webster, C.A.; Moncrieff, M.; Sherwood, V. The scope of nanaoparticle therapies for future metastatic melanoma treatment. Lancet Oncol. 2014, 15, e22–e32. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alhefdhi, A.Y.; Almalik, O.; Anwar, I.; Mahmood, R.; Mahasin, Z.; Al-Tweigeri, T. Primary oral malignant melanoma metastasis to the brain and breast: A case reports and literature review. Oncol. Lett. 2017, 14, 1275–1280. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin. Monograph. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar] [PubMed]
- Brito, A.F.; Ribeiro, M.; Abrantes, A.M.; Pires, A.S.; Teixo, R.J.; Tralhao, J.G.; Botelho, M.F. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr. Med. Chem. 2015, 22, 3025–3039. [Google Scholar] [CrossRef]
- Rafiq, R.A.; Quadri, A.; Nazir, L.A.; Peerzada, K.; Ganai, B.A.; Tasduq, S.A. A potent inhibitor of phosphoinositide 3-kinase (PI3K) and mitogen activated protein (MAP) kinase signaling, quercetin (3,3′,4′,5,7-pentahydroxyflavone) promotes cell death in ultraviote (UV)-B-lrradiated B16F10 melanoma cells. PLoS ONE 2015, 10, e0131253. [Google Scholar] [CrossRef]
- Devipriya, S.; Ganapathy, V.; Shymaladevi, C.S. Suppression of tumor growth and inmassion in 9,10 dimethyl benz(a) anthracene induced mammary carcinoma by the plant bioflavonoid quercetin. Chem. Biol. Interact. 2016, 162, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Alternat Med. 2011, 2011, 591356. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Wu, L.F.; Chen, W.K.; Wang, C.J.; Tseng, T.H. Inhibitory effects of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chem. Biol. Interact 2006, 160, 123–133. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Arunkumar, R.; Elumalai, P.; Sharmala, G.; Gunadharini, D.N.; Branudevi, S.; Krishnamoorthy, G.; Benson, C.S.; Arunakaran, J. Quercetin inhibits invasion, migration and signaling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3). Cell Biochem. Funct. 2011, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on natural polyphenols as anticancer agents for skin cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Tsuji, S. Significance of ganglioside-mediated glycosignal transduction in neuronal differentiation and development. Rrog Brain Res. 1994, 101, 119–126. [Google Scholar]
- Spiegel, S.; Fishman, P.H. Gangliosides as bimodal regulators of cell growth. Proc. Natl. Acad. Sci. USA 1987, 84, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef]
- Krengel, U.; Bousquet, P.A. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front. Immunol. 2014, 5, 99337. [Google Scholar] [CrossRef]
- Allende, M.L.; Proia, R.L. Lubricating cell signaling pathways with gangliosides. Curr. Opin. Struct. Biol. 2022, 12, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.L.; Olzomer, E.M.; Lolies, N.; Hoehn, K.L.; Wegner, M.S. Update on glycosphingolipids abundance in hepatocellular carcinoma. Int. J. Mol. Sci. 2022, 23, 4477. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Shi, Y.; Pan, X.; Feng, Q.; Wang, P.; Song, S.; Yang, L.; Yang, J. BR2 cell penetrating peptide effectively delivers anti-p21Ras scFv to tumor cells with ganglioside expression for therapy of ras-driven tumor. PLoS ONE 2022, 17, e0269084. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Z.; Lin, Z.; Yao, S.; Han, Y.; Fu, Q.; Liu, J. Label-free quantitative proteomics reveals the Steap3-Gm2a axis inhibiting the phagosomal escape of Listeria monocytogenes. Microbes Infect. 2022, 24, 104999. [Google Scholar] [CrossRef]
- Tsuchida, T.; Saxton, R.E.; Morton, D.L.; Irie, R.F. Gangliosides of human melanoma. Cancer 1989, 63, 1166–1174. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar] [PubMed]
- Aixinjueluo, W.; Furukawa, K.; Zhang, Q.; Hamamura, K.; Tokuda, N.; Yoshida, S.; Ueda, R.; Furukawa, K. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: Roles of anoikis. J. Biol. Chem. 2005, 280, 29828–29836. [Google Scholar] [CrossRef]
- Fan, T.; Chen, J.; Zhang, L.; Gao, P.; Hui, Y.; Xu, P.; Zhang, X.; Liu, H. Bit1 knockdown contributes to growth suppression as well as the decreases of migration and invasion abilities in esophageal squamous cell carcinoma via suppressing FAK-paxillin pathway. Mol. Cancer 2016, 15, 23. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.A.; Guan, J.L. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 1999, 4, 102–113. [Google Scholar] [CrossRef]
- Xia, H.; Nho, R.S.; Kahm, J.; Kleidon, J.; Henke, C.A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J. Biol. Chem. 2004, 279, 33024–33034. [Google Scholar] [CrossRef] [PubMed]
- Maria, R.; Lenti, L.; Malisan, F.; d‘Agostino, F.; Tomassini, B.; Zeuner, A.; Rippo, M.R.; Testi, R. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science 1997, 277, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Rodriguez-Walker, M.; Dewald, J.H.; Daniotti, J.L.; Delannoy, P. Gangliosides in cancer cell signaling. Prog. Mol. Biol. Transl. Sci. 2018, 156, 197–227. [Google Scholar]
- Groux-Degroote, S.; Guérardel, Y.; Delannoy, P. Gangliosides: Structures, biosynthesis, analysis, and roles in cancer. Chembiochem 2017, 18, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Hattori, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef]
- Queirolo, P.; Spagnolo, F. BRaf plus MEK-targeted drugs: A new standard of treatment for BRaf-mutant advanced melanoma. Cancer Metastasis Rev. 2017, 36, 35–42. [Google Scholar] [CrossRef]
- Mendoza, E.E.; Burd, R. Quercetin as a systemic chemopreventative agent: Structural and functional mechanisms. Mini Rev. Med. Chem. 2011, 11, 1216–1221. [Google Scholar]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ahn, A.; Chatterjee, A.; Eccles, M.R. The slow cycling phenotype: A growing problem for treatment resistance in melanoma. Mol. Cancer Ther. 2017, 16, 1002–1009. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Piantelli, M.; Rossi, C.; Lezzi, M.; La Sorda, R.L.; Lacobelli, S.; Alberti, S.; Natali, P.G. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J. Cell Physiol. 2006, 207, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rosner, K.; Ropke, C.; Pless, V.; Skovgaard, G.L. Late type apoptosis and apoptosis free lenthal effect of quercetin in human melanoma. Biosci. Biotechnol. Biochem. 2006, 70, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; Baliga, M.S.; Elmets, C.A.; Katiyar, S.K. Grape seed proanthocyanidins induce apoptosis through p53, Bax, and caspase 3 pathways. Neoplasia 2005, 7, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.R.; Kim, J.; Na, J.C.; Han, W.K. Mitochondrial metabolic reprogramming by SIRT3 regulation ameliorates drug resistance in renal cell carcinoma. PLoS ONE 2022, 17, e0269432. [Google Scholar] [CrossRef] [PubMed]
- Portoukalian, J.; Zwingelstein, G.; Dore, J.F. Lipid composition of human malignant melanoma tumors at various levels of malignant growth. Eur. J. Biochem. 1979, 94, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Lloyd, K.O. Gangliosides in Melanoma. In Human Melanoma: From Basic Research to Clinical Application; Ferrone, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 15–30. [Google Scholar]
- Ju, W.S.; Seo, S.Y.; Mun, S.-E.; Kim, K.; Yu, J.O.; Ryu, J.-S.; Kim, J.-S.; Choo, Y.-K. 7,8-Dihydroxyflavone induces mitochondrial apoptosis and down-regulates the expression of ganglioside GD3 in malignant melanoma cells. Discov. Oncol. 2023, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Tilghman, R.W.; Parsons, J.T. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin. Cancer Biol. 2008, 18, 45–52. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Schraw, W.; Richmond, A. Melanoma growth stimulatory activity signaling through the class II interleukin-8 receptor enhances the tyrosin phosphorylation of Crk-associated substrate, p130, and a 70-kilodalton protein. Biochemistry 1995, 34, 13760–13767. [Google Scholar] [CrossRef]
- Prockop, D.J.; Kivirikko, K. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.H.; Seo, Y.N.; Lee, J.H.; Park, S.J.; Cho, Y.H.; Kim, J.S.; Kim, S.U.; Choo, Y.K. GM1 induced the inflammatory response related to the Raf-1/MEK1/2ERK1/2 pathway in co-cultured of pig mesenchymal stem cells with RAW264.7. Anim. Cell. Syst. 2018, 22, 157–164. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, S.Y.; Ju, W.S.; Kim, K.; Kim, J.; Yu, J.O.; Ryu, J.-S.; Kim, J.-S.; Lee, H.-A.; Koo, D.-B.; Choo, Y.-K. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 5146. https://doi.org/10.3390/ijms25105146
Seo SY, Ju WS, Kim K, Kim J, Yu JO, Ryu J-S, Kim J-S, Lee H-A, Koo D-B, Choo Y-K. Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells. International Journal of Molecular Sciences. 2024; 25(10):5146. https://doi.org/10.3390/ijms25105146
Chicago/Turabian StyleSeo, Sang Young, Won Seok Ju, Kyongtae Kim, Juhwan Kim, Jin Ok Yu, Jae-Sung Ryu, Ji-Su Kim, Hyun-A Lee, Deog-Bon Koo, and Young-Kug Choo. 2024. "Quercetin Induces Mitochondrial Apoptosis and Downregulates Ganglioside GD3 Expression in Melanoma Cells" International Journal of Molecular Sciences 25, no. 10: 5146. https://doi.org/10.3390/ijms25105146




