Unveiling a Microexon Switch: Novel Regulation of the Activities of Sugar Assimilation and Plant-Cell-Wall-Degrading Xylanases and Cellulases by Xlr2 in Trichoderma virens
Abstract
:1. Introduction
2. Results
2.1. Xlr2 Is a Novel Zn(II)2Cys6 Transcription Factor Conserved in Filamentous Fungi
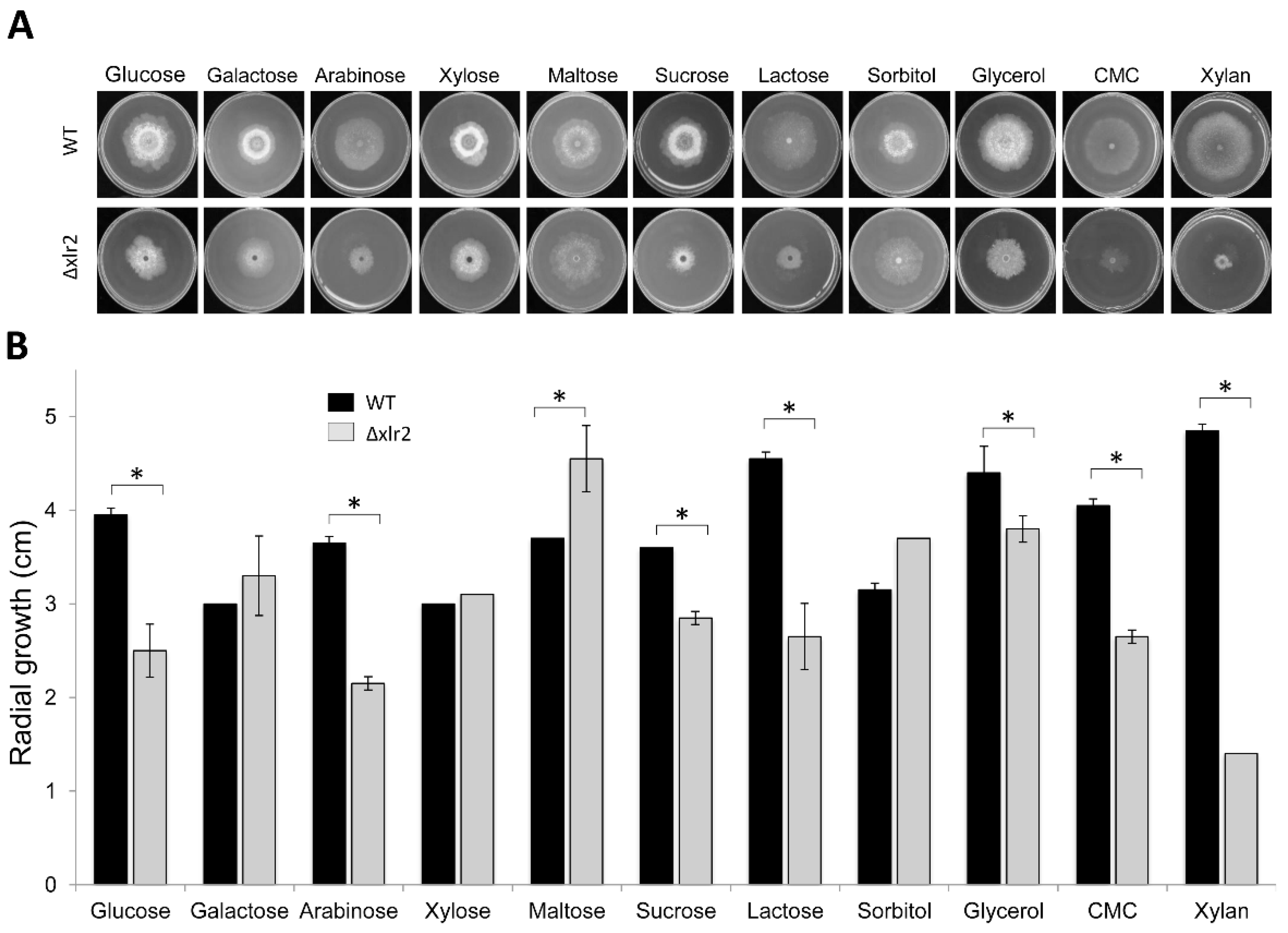
2.2. Xlr2 Is Involved in Morphology and Sugar Assimilation in T. virens
2.3. Xlr2 Transcription Factor Partially Regulates PCWDE Activity in T. virens
2.4. A Microexon in Xlr2 Is Conserved in the Trichoderma Genus
2.5. Differential Expression of the Microexon in Xlr2 Represents a Novel Regulatory Mechanism in Sugar Assimilation and Cell-Wall-Degrading Enzymes in Trichoderma
2.6. OEXlr2 Regulates Cellulase and Xylanase Biosynthesis in Trichoderma virens under Solid-State and Submerged Fermentation
3. Discussion
3.1. Identification and Characterization of a Novel and Conserved Zn(II)2-C6 Transcription Activating Factor from T. virens
3.2. The Transcription Activator Xlr2 Is Conserved in Trichoderma
3.3. Xlr2 Is Involved in the Regulation of Xylanase and Cellulase Activity
3.4. The Regulation of PCWDEs via Fungal Perceptions of Plant Cell Wall Polysaccharides
3.5. Role of the Long and Short Isoforms of Xlr2 and the "Switch-like" Microexon
3.6. Carbohydrate Assimilation in the Long and Short Xlr2 Isoforms
3.7. Overexpression of the Long and Short Xlr2 Isoforms Affects Catabolism
3.8. The Xlr2 Microexon Regulates Cellulase and Xylase Activity during SmF and SSF
3.9. Cellulase and Xylanase Activity Is Affected by the Fermentation Technique
4. Materials and Methods
4.1. Fungal Strains
4.2. Growth Conditions
4.3. Construction of Plasmids
4.4. Protoplast Preparation and Transformation
4.5. Gene Expression Studies
4.6. Xylanase and Cellulase Activity in SSF and SmF
4.7. Obtaining Enzymatic Extracts
4.8. Analytical Techniques
4.9. RNA-Seq Analysis of the Alternative Splicing
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Cellular, genomic and metabolic complexity. Biol. Rev. 2020, 95, 1198–1232. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.-T.; Jeon, J.; Lee, Y.-H. Fungal plant cell wall-degrading enzyme database: A platform for comparative and evolutionary genomics in fungi and Oomycetes. BMC Genom. 2013, 14, S7. [Google Scholar] [CrossRef]
- Aro, N.; Pakula, T.; Penttilä, M. Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol. Rev. 2005, 29, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.A.; Horta, M.A.C.; Ferreira Filho, J.A.; Murad, N.F.; de Souza, A.P. The synergistic actions of hydrolytic genes reveal the mechanism of Trichoderma harzianum for cellulose degradation. J. Biotechnol. 2021, 334, 1–10. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Van Peij, N.N.; Visser, J.; De Graaff, L.H. Isolation and analysis of xlnR, encoding a transcriptional activator co-ordinating xylanolytic expression in Aspergillus niger. Mol. Microbiol. 1998, 27, 131–142. [Google Scholar] [CrossRef]
- Stricker, A.R.; Mach, R.L.; De Graaff, L.H. Regulation of transcription of cellulases-and hemicellulases-encoding genes in Aspergillus niger and Hypocrea jecorina (Trichoderma reesei). Appl. Microbiol. Biotechnol. 2008, 78, 211–220. [Google Scholar] [CrossRef]
- Noguchi, Y.; Sano, M.; Kanamaru, K.; Ko, T.; Takeuchi, M.; Kato, M.; Kobayashi, T. Genes regulated by AoXlnR, the xylanolytic and cellulolytic transcriptional regulator, in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2009, 85, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tian, C.; Diamond, S.; Glass, N.L. Deciphering transcriptional regulatory mechanisms associated with hemicellulose degradation in Neurospora crassa. Eukaryot. Cell 2012, 11, 482–493. [Google Scholar] [CrossRef]
- Stricker, A.R.; Grosstessner-Hain, K.; Würleitner, E.; Mach, R.L. Xyr1 (xylanase regulator 1) regulates both the hydrolytic enzyme system and D-xylose metabolism in Hypocrea jecorina. Eukaryot. Cell 2006, 5, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, M.; Valkonen, M.J.; Westerholm-Parvinen, A.; Aro, N.; Arvas, M.; Vitikainen, M.; Penttilä, M.; Saloheimo, M.; Pakula, T.M. Screening of candidate regulators for cellulase and hemicellulase production in Trichoderma reesei and identification of a factor essential for cellulase production. Biotechnol. Biofu. 2014, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Stricker, A.R.; Steiger, M.G.; Mach, R.L. Xyr1 receives the lactose induction signal and regulates lactose metabolism in Hypocrea jecorina. FEBS Lett. 2007, 581, 3915–3920. [Google Scholar] [CrossRef] [PubMed]
- Curry-Hyde, A.; Chen, B.J.; Mills, J.D.; Janitz, M. Microexons: Novel regulators of the transcriptome. J. Hum. Transcr. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- DeMarco, R.; Mathieson, W.; Manuel, S.J.; Dillon, G.P.; Curwen, R.S.; Ashton, P.D.; Ivens, A.C.; Berriman, M.; Verjovski-Almeida, S.; Wilson, R.A. Protein variation in blood-dwelling schistosome worms generated by differential splicing of micro-exon gene transcripts. Geno. Res. 2010, 20, 1112–1121. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.-L. Microexons go big. Cell 2014, 159, 1488–1489. [Google Scholar] [CrossRef]
- Volfovsky, N.; Haas, B.J.; Salzberg, S.L. Computational discovery of internal micro-exons. Genome Res. 2003, 13, 1216–1221. [Google Scholar] [CrossRef]
- Garcia, K.; Gloyn, A.L. Small but mighty: Microexons in glucose homeostasis. Trend. Genet. 2023, 39, 526–527. [Google Scholar] [CrossRef]
- Juan-Mateu, J.; Bajew, S.; Miret-Cuesta, M.; Íñiguez, L.P.; Lopez-Pascual, A.; Bonnal, S.; Atla, G.; Bonàs-Guarch, S.; Ferrer, J.; Valcárcel, J.; et al. Pancreatic microexons regulate islet function and glucose homeostasis. Nat. Metab. 2023, 5, 219–236. [Google Scholar] [CrossRef]
- Romfo, C.M.; Alvarez, C.J.; van Heeckeren, W.J.; Webb, C.J.; Wise, J.A. Evidence for splice site pairing via intron definition in Schizosaccharomyces pombe. Mol. Cell. Biol. 2000, 20, 7955–7970. [Google Scholar] [CrossRef]
- Kostrub, C.; Al-Khodairy, F.; Ghazizadeh, H.; Carr, A.; Enoch, T. Molecular analysis of hus1+, a fission yeast gene required for SM and DNA damage checkpoints. Molec. Gen. Genet. MGG 1997, 254, 389–399. [Google Scholar] [PubMed]
- Bumpus, J.A.; Trax, M.; Reisdorph, A.; Boyd, C.; Gilbert, D.; Techau, S.; Ventullo, R.M. An in silico analysis of cytochrome c from Phanerochaete chrysosporium: Its amino acid sequence and characterization of gene structural elements. In Silico Biol. 2008, 8, 1–13. [Google Scholar] [PubMed]
- Doddapaneni, H.; Chakraborty, R.; Yadav, J.S. Genome-wide structural and evolutionary analysis of the P450 monooxygenase genes (P450ome) in the white rot fungus Phanerochaete chrysosporium: Evidence for gene duplications and extensive gene clustering. BMC Genom. 2005, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Kummasook, A.; Kummasook, A.; Pongpom, P.; Kummasook, A.; Pongpom, P.; Vanittanakom, N. Cloning, characterization and differential expression of an hsp70 gene from the pathogenic dimorphic fungus, Penicillium marneffei. DNA Seq. 2007, 18, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Iotti, M.; Rubini, A.; Tisserant, E.; Kholer, A.; Paolocci, F.; Zambonelli, A. Self/nonself recognition in Tuber melanosporum is not mediated by a heterokaryon incompatibility system. Fungal. Biol. 2012, 116, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Dvinge, H. Regulation of alternative mRNA splicing: Old players and new perspectives. FEBS Lett. 2018, 592, 2987–3006. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, K.; Szafranski, K.; Pohl, M.; Voigt, K.; Petzold, A.; Schuster, S. Fungal alternative splicing is associated with multicellular complexity and virulence: A genome-wide multi-species study. DNA Res. 2014, 21, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ustianenko, D.; Weyn-Vanhentenryck, S.M.; Zhang, C. Microexons: Discovery, regulation, and function. Wiley Interdiscip. Rev. RNA 2017, 8, e1418. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Magen, A.; Ast, G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007, 35, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Irimia, M.; Weatheritt, R.J.; Ellis, J.D.; Parikshak, N.N.; Gonatopoulos-Pournatzis, T.; Babor, M.; Quesnel-Vallières, M.; Tapial, J.; Raj, B.; O’Hanlon, D. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell 2014, 159, 1511–1523. [Google Scholar] [CrossRef]
- Parras, A.; Anta, H.; Santos-Galindo, M.; Swarup, V.; Elorza, A.; Nieto-González, J.L.; Picó, S.; Hernández, I.H.; Díaz-Hernández, J.I.; Belloc, E. Autism-like phenotype and risk gene mRNA deadenylation by CPEB4 mis-splicing. Nature 2018, 560, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Gen. Biol. 2011, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Multifunctional fungal plant symbionts: New tools to enhance plant growth and productivity. New Phytolog. 2011, 189, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From’omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Lawry, R. Cross-Communication between Trichoderma and Plants during Root Colonisation. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2016. [Google Scholar]
- Morán-Diez, M.E.; Trushina, N.; Lamdan, N.L.; Rosenfelder, L.; Mukherjee, P.K.; Kenerley, C.M.; Horwitz, B.A. Host-specific transcriptomic pattern of Trichoderma virens during interaction with maize or tomato roots. BMC Genom. 2015, 16, 8. [Google Scholar] [CrossRef]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M.; Mendoza-Mendoza, A. The apoplastic secretome of Trichoderma virens during interaction with maize roots shows an inhibition of plant defence and scavenging oxidative stress secreted proteins. Front. Plant Sci. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Lamdan, N.-L.; Shalaby, S.; Ziv, T.; Kenerley, C.M.; Horwitz, B.A. Secretome of Trichoderma Interacting With Maize Roots: Role in Induced Systemic Resistance. Mol. Cell Proteom. 2015, 14, 1054–1063. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.J.; Grujic, M.; Cai, F.; Pourmehdi, S. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, H.; Deng, W.; Li, T. Genome-Wide Analysis of the Zn(II)₂Cys₆ Zinc Cluster-Encoding Gene Family in Tolypocladiumguangdongense and Its Light-Induced Expression. Genes 2019, 10, 179. [Google Scholar] [CrossRef]
- Shelest, E. Transcription Factors in Fungi: TFome Dynamics, Three Major Families, and Dual-Specificity TFs. Front. Genet. 2017, 8, 53. [Google Scholar] [CrossRef]
- Todd, R.B.; Zhou, M.; Ohm, R.A.; Leeggangers, H.A.; Visser, L.; De Vries, R.P. Prevalence of transcription factors in ascomycete and basidiomycete fungi. BMC Genom. 2014, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Seiboth, B.; Herold, S.; Kubicek, C.P. Metabolic engineering of inducer formation for cellulase and hemicellulase gene expression in Trichoderma reesei. Subcell. Biochem. 2012, 64, 367–390. [Google Scholar] [PubMed]
- Mendoza-Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.A.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Traven, A.; Jelicic, B.; Sopta, M. Yeast Gal4: A transcriptional paradigm revisited. EMBO Rep. 2006, 7, 496–499. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, gkae268. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.M.; Drabenstot, S.D.; Buchanan, K.L.; Lai, H.; Zhu, H.; Dyer, D.W.; Roe, B.A.; Murphy, J.W. Introns and splicing elements of five diverse fungi. Eukaryot. Cell 2004, 3, 1088–1100. [Google Scholar] [CrossRef]
- Shelest, E. Transcription factors in fungi. FEMS Microbiol. Lett. 2008, 286, 145–151. [Google Scholar] [CrossRef]
- Raulo, R.; Kokolski, M.; Archer, D.B. The roles of the zinc finger transcription factors XlnR, ClrA and ClrB in the breakdown of lignocellulose by Aspergillus niger. Amb. Express. 2016, 6, 5. [Google Scholar] [CrossRef]
- Battaglia, E.; Visser, L.; Nijssen, A.; van Veluw, G.; Wösten, H.; de Vries, R. Analysis of regulation of pentose utilisation in Aspergillus niger reveals evolutionary adaptations in Eurotiales. Stud. Mycol. 2011, 69, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.E.; Gruben, B.S.; Battaglia, E.; Wiebenga, A.; Majoor, E.; de Vries, R.P. Genetic interaction of Aspergillus nidulans galR, xlnR and araR in regulating D-galactose and L-arabinose release and catabolism gene expression. PLoS ONE 2015, 10, e0143200. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Zhou, M.; de Vries, R.P. The transcriptional activators AraR and XlnR from Aspergillus niger regulate expression of pentose catabolic and pentose phosphate pathway genes. Res. Microbiol. 2014, 165, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Aro, N.; Saloheimo, A.; Ilmén, M.; Penttilä, M. ACEII, a novel transcriptional activator involved in regulation of cellulase and xylanase genes of Trichoderma reesei. J. Biol. Chem. 2001, 276, 24309–24314. [Google Scholar] [CrossRef]
- Zeilinger, S.; Ebner, A.; Marosits, T.; Mach, R.; Kubicek, C. The Hypocrea jecorina HAP 2/3/5 protein complex binds to the inverted CCAAT-box (ATTGG) within the cbh2 (cellobiohydrolase II-gene) activating element. Mol. Genet. Genom. 2001, 266, 56–63. [Google Scholar] [CrossRef]
- Derntl, C.; Gudynaite-Savitch, L.; Calixte, S.; White, T.; Mach, R.L.; Mach-Aigner, A.R. Mutation of the Xylanase regulator 1 causes a glucose blind hydrolase expressing phenotype in industrially used Trichoderma strains. Biotechnol. Biofuels 2013, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Derntl, C.; Rassinger, A.; Srebotnik, E.; Mach, R.L.; Mach-Aigner, A.R. Xpp1 regulates the expression of xylanases, but not of cellulases in Trichoderma reesei. Biotechnol. Biofuels 2015, 8, 112. [Google Scholar] [CrossRef]
- Benocci, T.; Aguilar-Pontes, M.V.; Zhou, M.; Seiboth, B.; de Vries, R.P. Regulators of plant biomass degradation in ascomycetous fungi. Biotechnol. Biofuels 2017, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Klaubauf, S.; Vallet, J.; Ribot, C.; Lebrun, M.-H.; De Vries, R.P. Xlr1 is involved in the transcriptional control of the pentose catabolic pathway, but not hemi-cellulolytic enzymes in Magnaporthe oryzae. Fungal Genet. Biol. 2013, 57, 76–84. [Google Scholar] [CrossRef]
- Serebryanyi, V.; Sinitsyna, O.; Fedorova, E.; Okunev, O.; Bekkarevich, O.; Sokolova, L.; Vavilova, E.; Vitetsky, Y.P.; Sinitsyn, A. Synthesis of xylanolytic enzymes and other carbohydrases by the fungus Penicillium canescens: Transformants with an altered copy number of the gene xln R and an encoding transcriptional activator. Appl. Biochem. Microbiol. 2006, 42, 584–591. [Google Scholar] [CrossRef]
- Mach-Aigner, A.R.; Grosstessner-Hain, K.; Poças-Fonseca, M.J.; Mechtler, K.; Mach, R.L. From an electrophoretic mobility shift assay to isolated transcription factors: A fast genomic-proteomic approach. BMC Genom. 2010, 11, 644. [Google Scholar] [CrossRef]
- Battaglia, E.; Hansen, S.F.; Leendertse, A.; Madrid, S.; Mulder, H.; Nikolaev, I.; de Vries, R.P. Regulation of pentose utilisation by AraR, but not XlnR, differs in Aspergillus nidulans and Aspergillus niger. Appl. Microbiol. Biotechnol. 2011, 91, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Herold, S.; Bischof, R.; Metz, B.; Seiboth, B.; Kubicek, C.P. Xylanase gene transcription in Trichoderma reesei is triggered by different inducers representing different hemicellulosic pentose polymers. Eukaryot. Cell 2013, 12, 390–398. [Google Scholar] [CrossRef]
- Carle-Urioste, J.C.; Escobar-Vera, J.; El-Gogary, S.; Henrique-Silva, F.; Torigoi, E.; Crivellaro, O.; Herrera-Estrella, A.; El-Dorry, H. Cellulase induction in Trichoderma reesei by cellulose requires its own basal expression. J. Biol. Chem. 1997, 272, 10169–10174. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Chen, X.; Hehl, R.; Karas, H.; Liebich, I.; Matys, V.; Meinhardt, T.; Prüß, M.; Reuter, I.; Schacherer, F. TRANSFAC: An integrated system for gene expression regulation. Nucleic Acids Res. 2000, 28, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.I.; Sanchez-Pulido, L.; Haerty, W.; Ponting, C.P. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Res. 2015, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L. The Pfam protein families database. Nucleic Acids Res. 2004, 32 (Suppl. S1), D138–D141. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Larochelle, M.; Turcotte, B. A fungal family of transcriptional regulators: The zinc cluster proteins. Microbiol. Mol. Biol. R. 2006, 70, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Seiboth, B.; Metz, B. Fungal arabinan and L-arabinose metabolism. Appl. Microbiol. Biotechnol. 2011, 89, 1665–1673. [Google Scholar] [CrossRef]
- Mojzita, D.; Herold, S.; Metz, B.; Seiboth, B.; Richard, P. L-xylo-3-hexulose reductase is the missing link in the oxidoreductive pathway for D-galactose catabolism in filamentous fungi. J. Biol. Chem. 2012, 287, 26010–26018. [Google Scholar] [CrossRef]
- Nogawa, M.; Goto, M.; Okada, H.; Morikawa, Y. L-Sorbose induces cellulase gene transcription in the cellulolytic fungus Trichoderma reesei. Curr. Genet. 2001, 38, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ilmen, M.; Saloheimo, A.; Onnela, M.-L.; Penttilä, M.E. Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl. Environ. Microbiol. 1997, 63, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Rayment, I.; Thoden, J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 2003, 278, 43885–43888. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.A. The Leloir pathway: A mechanistic imperative for three enzymes to change the stereochemical configuration of a single carbon in galactose. FASEB J. 1996, 10, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Klaubauf, S.; Zhou, M.; Lebrun, M.H.; de Vries, R.P.; Battaglia, E. A novel l-arabinose-responsive regulator discovered in the rice-blast fungus Pyricularia oryzae (Magnaporthe oryzae). FEBS Lett. 2016, 590, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, C.; Bååth, J.A.; Seiboth, B.; Kubicek, C.P. Systems analysis of lactose metabolism in Trichoderma reesei identifies a lactose permease that is essential for cellulase induction. PLoS ONE 2013, 8, e62631. [Google Scholar] [CrossRef]
- Da Silva Delabona, P.; Pirota, R.D.B.; Codima, C.A.; Tremacoldi, C.R.; Rodrigues, A.; Farinas, C.S. Using Amazon forest fungi and agricultural residues as a strategy to produce cellulolytic enzymes. Biom. Bioenergy 2012, 37, 243–250. [Google Scholar] [CrossRef]
- Zeilinger, S.; Mach, R. Xylanolytic enzymes of Trichoderma reesei: Properties and regulation of expression. Curr. Top. Cer. Chem. 1998, 1, 27–35. [Google Scholar]
- Dos Santos Castro, L.; de Paula, R.G.; Antonieto, A.C.; Persinoti, G.F.; Silva-Rocha, R.; Silva, R.N. Understanding the role of the master regulator XYR1 in Trichoderma reesei by global transcriptional analysis. Front. Microbiol. 2016, 7, 175. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Gong, Y.; Yu, S.; Liu, G. Enhancing xylanase production in the thermophilic fungus Myceliophthora thermophila by homologous overexpression of Mtxyr1. J. Ind. Microbiol. Biotechnol. 2015, 42, 1233–1241. [Google Scholar] [CrossRef]
- Dos Santos Castro, L.; Antoniêto, A.C.C.; Pedersoli, W.R.; Silva-Rocha, R.; Persinoti, G.F.; Silva, R.N. Expression pattern of cellulolytic and xylanolytic genes regulated by transcriptional factors XYR1 and CRE1 are affected by carbon source in Trichoderma reesei. Gen. Express. Pat. 2014, 14, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Han, J.; Li, Y.; Liu, J.; Gan, L.; Long, M. Promoting cellulase and hemicellulase production from Trichoderma orientalis EU7-22 by overexpression of transcription factors Xyr1 and Ace3. Bioresour. Technol. 2020, 296, 122355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Wu, C.; Liu, P.; Wang, W.; Wei, D. The transcription factor ACE3 controls cellulase activities and lactose metabolism via two additional regulators in the fungus Trichoderma reesei. J. Biol. Chem. 2019, 294, 18435–18450. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Montiel-González, A.M.; Fernández, F.J.; Viniegra-González, G.; Loera, O. Invertase production on solid-state fermentation by Aspergillus niger strains improved by parasexual recombination. Appl. Biochem. Biotechnol. 2002, 102, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q. 2008, 22, 49–70. [Google Scholar]
- Garg, U.K.; Kaur, M.; Garg, V.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazar. Mater. 2007, 140, 60–68. [Google Scholar] [CrossRef]
- De A. Ximenes, F.; de Paula Silveira, F.Q.; Filho, E.X.F. Production of β-Xylosidase Activity by Trichoderma harzianum Strains. Curr. Microbiol. 1996, 33, 71–77. [Google Scholar] [CrossRef]
- Rezende, M.I.; Barbosa, A.d.M.; Vasconcelos, A.F.D.; Endo, A.S. Xylanase production by Trichoderma harzianum rifai by solid state fermentation on sugarcane bagasse. Braz. J. Microbiol. 2002, 33, 67–72. [Google Scholar] [CrossRef]
- Nair, S.G.; Sindhu, R.; Shashidhar, S. Fungal xylanase production under solid state and submerged fermentation conditions. Afr. J. Microbiol. Res. 2008, 2, 82–86. [Google Scholar]
- Lee, S.J.; Jang, Y.S.; Lee, Y.M.; Lee, J.J.; Lee, H.B.; Kim, G.H.; Kim, J.J. Rice straw-decomposing fungi and their cellulolytic and xylanolytic enzymes. J. Microbiol. Biotechnol. 2011, 21, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Al-Malki, A.L.; Khan, J.A.; Kabli, S.A.; Al-Garni, S.M. Solid state production of polygalacturonase and xylanase by Trichoderma species using cantaloupe and watermelon rinds. J. Microbiol. Biotechnol. 2013, 51, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elmagd, H.I. Optimization and biochemical characterization of exracellular xylanase from Trichoderma harzianum MH-20 under solid state fermentation. Life Sci. J 2014, 11, 188–195. [Google Scholar]
- Haq, I.; Shahzadi, K.; Hameed, U.; Javed, M.; Qadeer, M. Solid state fermentation of cellulases by locally isolated Trichoderma harzianum for the exploitation of agricultural byproducts. Pak. J. Biol. Sci. 2006, 9, 1779–1782. [Google Scholar] [CrossRef]
- Mangalanayaki, R.; Madhavan, S. Cellulase production by Trichoderma harzianum and Fusarium oxysporum under solid state fermentation. World J. Pharm. Pharm. Sci. 2015, 4, 1822–1828. [Google Scholar]
- Nanjundaswamy, A.; Okeke, B.C. Comprehensive optimization of culture conditions for production of biomass-hydrolyzing enzymes of Trichoderma SG2 in submerged and solid-state fermentation. Appl. Biochem. Biotechnol. 2020, 191, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Pawar, S.; Rathod, V. Optimised cellulase production from fungal co-culture of Trichoderma reesei NCIM 1186 and Penicillium citrinum NCIM 768 under solid state fermentation. J. Environ. Chem. Eng. 2020, 8, 103958. [Google Scholar] [CrossRef]
- Toscano, L.; Montero, G.; Stoytcheva, M.; Gochev, V.; Cervantes, L.; Campbell, H.; Zlatev, R.; Valdez, B.; Pérez, C.; Gil-Samaniego, M. Lipase production through solid-state fermentation using agro-industrial residues as substrates and newly isolated fungal strains. Biotechnol. Biotechnol. Equip. 2013, 27, 4074–4077. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Christensen, T.M.; Mikkelsen, J.D. New polygalacturonases from Trichoderma reesei: Characterization and their specificities to partially methylated and acetylated pectins. Carbohydr. Res. 2003, 338, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. A convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Téllez-Téllez, M.; Fernández, F.; Montiel-González, A.; Sánchez, C.; Díaz-Godínez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Nogueira-López, G.; Padilla-Arizmendi, F.; Inwood, S.; Lyne, S.; Steyaert, J.M.; Nieto-Jacobo, M.F.; Stewart, A.; Mendoza-Mendoza, A. TrichoGate: An Improved Vector System for a Large Scale of Functional Analysis of Trichoderma Genes. Front. Microbiol. 2019, 10, 2794. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Meth. 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Williams, L.J.; Abdi, H. Fisher’s least significant difference (LSD) test. Encyclop. Res. Des. 2010, 218, 840–853. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Casasola, C.C.; Nieto-Jacobo, M.F.; Soares, A.; Padilla-Padilla, E.A.; Anducho-Reyes, M.A.; Brown, C.; Soth, S.; Esquivel-Naranjo, E.U.; Hampton, J.; Mendoza-Mendoza, A. Unveiling a Microexon Switch: Novel Regulation of the Activities of Sugar Assimilation and Plant-Cell-Wall-Degrading Xylanases and Cellulases by Xlr2 in Trichoderma virens. Int. J. Mol. Sci. 2024, 25, 5172. https://doi.org/10.3390/ijms25105172
Castañeda-Casasola CC, Nieto-Jacobo MF, Soares A, Padilla-Padilla EA, Anducho-Reyes MA, Brown C, Soth S, Esquivel-Naranjo EU, Hampton J, Mendoza-Mendoza A. Unveiling a Microexon Switch: Novel Regulation of the Activities of Sugar Assimilation and Plant-Cell-Wall-Degrading Xylanases and Cellulases by Xlr2 in Trichoderma virens. International Journal of Molecular Sciences. 2024; 25(10):5172. https://doi.org/10.3390/ijms25105172
Chicago/Turabian StyleCastañeda-Casasola, Cynthia Coccet, María Fernanda Nieto-Jacobo, Amanda Soares, Emir Alejandro Padilla-Padilla, Miguel Angel Anducho-Reyes, Chris Brown, Sereyboth Soth, Edgardo Ulises Esquivel-Naranjo, John Hampton, and Artemio Mendoza-Mendoza. 2024. "Unveiling a Microexon Switch: Novel Regulation of the Activities of Sugar Assimilation and Plant-Cell-Wall-Degrading Xylanases and Cellulases by Xlr2 in Trichoderma virens" International Journal of Molecular Sciences 25, no. 10: 5172. https://doi.org/10.3390/ijms25105172






