Genome-Wide Profile of Mutations Induced by Carbon Ion Beam Irradiation of Dehulled Rice Seeds
Abstract
:1. Introduction
2. Results
2.1. Summarized Information of CIB-Induced Mutations
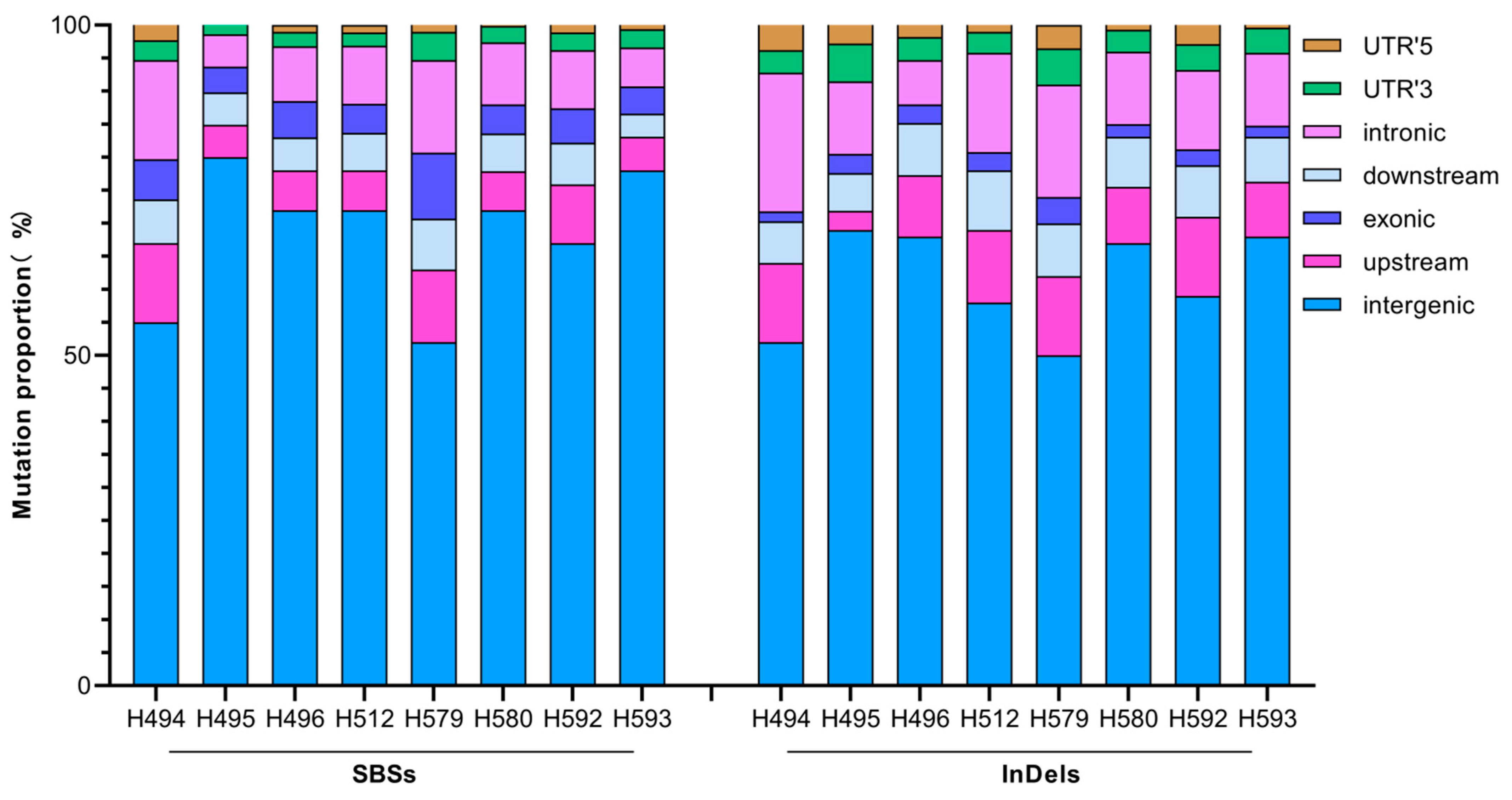
2.2. Frequency and Distribution of Mutations for CIB Irradiation
2.2.1. Distribution of Mutations on Chromosomes
2.2.2. Theoretical Mutation Frequency of the M1 Generation
2.3. Characteristics of Mutations Induced by CIB Irradiation
2.4. Effect of CIB-Induced Mutation on Gene Function
2.5. Verification of the Identified Mutation Sites
3. Discussion
3.1. The Mutation Rate of Dehulled Rice Seeds Induced by Carbon Ion Beam
3.2. SBSs and InDels Are the Two Main Mutation Types Occurring under CIB Irradiation
3.3. Gene Function-Affecting Mutations Provide Some Clues to Gene Identification
4. Materials and Methods
4.1. Plant Material, Mutagenesis, and Mutant Screening
4.2. Whole-Genome Sequencing and Clean Read Filtering
4.3. SNP and InDel Identification
Variant Annotation and Gene Function Prediction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Luo, W.; Zhang, J.; Yan, X.; Zhou, L. Genome-Wide Comparisons of Mutations Induced by Carbon-Ion Beam and Gamma-Rays Irradiation in Rice via Resequencing Multiple Mutants. Front. Plant Sci. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, S.; Schneeberger, K.; Lucas-Lledo, J.I.; Warthmann, N.; Clark, R.M.; Shaw, R.G.; Weigel, D.; Lynch, M. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science 2010, 327, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Shikazono, N.; Hase, Y. Studies on Biological Effects of Ion Beams on Lethality, Molecular Nature of Mutation, Mutation Rate, and Spectrum of Mutation Phenotype for Mutation Breeding in Higher Plants. J. Radiat. Res. 2010, 3, 223. [Google Scholar] [CrossRef] [PubMed]
- Kazama, Y.; Hirano, T.; Saito, H.; Liu, Y.; Ohbu, S.; Hayashi, Y.; Abe, T. Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Luo, S.; Li, X.; Yang, J.; Cui, T.; Li, W.; Yu, L.; Feng, H.; Chen, H.; Mu, J.; et al. Identification of Substitutions and Small Insertion-Deletions Induced by Carbon-Ion Beam Irradiation in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1851. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Lshimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef] [PubMed]
- Ichitani, K.; Yamaguchi, D.; Taura, S.; Fukutoku, Y.; Onoue, M.; Shimizu, K.; Hashimoto, F.; Sakata, Y.; Sato, M. Genetic analysis of ion-beam induced extremely late heading mutants in rice. Breed. Sci. 2014, 64, 222–230. [Google Scholar] [CrossRef]
- Oono, Y.; Ichida, H.; Morita, R.; Nozawa, S.; Satoh, K.; Shimizu, A.; Abe, T.; Kato, H.; Hase, Y. Genome sequencing of ion-beam-induced mutants facilitates detection of candidate genes responsible for phenotypes of mutants in rice. Mutat. Res. 2020, 821, 111691. [Google Scholar] [CrossRef]
- Takano, N.; Takahashi, Y.; Yamamoto, M.; Teranishi, M.; Yamaguchi, H.; Sakamoto, A.; Hase, Y.; Fujisawa, H.; Wu, J.; Matsumoto, T. Isolation of a novel UVB-tolerant rice mutant obtained by exposure to carbon-ion beams. J. Radiat. Res. 2013, 4, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Mohd, Y.; Harun, A.; Faiz, A.; Sobri, H.; Yusof, S. Screening of phenotypic performance, drought, and salinity tolerance in the mutagenized population of Oryza sativa cv. MR219 generated through ion beam irradiation. Int. J. Agric. Technol. 2019, 17, 1735–1752. [Google Scholar]
- Tabassum, R.; Dosaka, T.; Ichida, H.; Morita, R.; Katsube-Tanaka, T. FLOURY ENDOSPERM11-2 encodes plastid HSP70-2 involved with temperature-dependent chalkiness of rice (Oryza sativa L.) grains. Plant J. 2020, 103, 604–616. [Google Scholar] [CrossRef]
- Kazama, Y.; Saito, H.; Miyagai, M.; Takehisa, H.; Mii, M. Effect of heavy ion-beam irradiation on plant growth and mutation induction in Nicotiana tabacum. Plant Biotechnol. 2008, 25, 105–111. [Google Scholar] [CrossRef]
- Lee, M.B.; Kim, D.Y.; Seo, Y.W. Identification of candidate genes for the seed coat colour change in a Brachypodium distachyon mutant induced by gamma radiation using whole-genome re-sequencing. Genome 2017, 60, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shimizu, A.; Nishio, T.; Tsutsumi, N.; Kato, H. Comparison and Characterization of Mutations Induced by Gamma-Ray and Carbon-Ion Irradiation in Rice (Oryza sativa L.) Using Whole-Genome Resequencing. G3-Genes Genomes Genet. 2019, 9, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Huang, J.; Fu, H.; Zhou, L.; Furusawa, Y.; Shu, Q. Identification and characterization of inheritable structural variations induced by ion beam radiations in rice. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2021, 823, 111757. [Google Scholar] [CrossRef] [PubMed]
- Ichida, H.; Morita, R.; Shirakawa, Y.; Hayashi, Y.; Abe, T. Targeted exome sequencing of unselected heavy-ion beam-irradiated populations reveals less-biased mutation characteristics in the rice genome. Plant J. Cell Mol. Biol. 2018, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Mccallum, C.M.; Comai, L.; Henikoff, G.S. Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol. 2000, 123, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jain, R.; Chern, M.; Pham, N.T.; Martin, J.A.; Wei, T.; Schackwitz, W.S.; Lipzen, A.M.; Duong, P.Q.; Jones, K.C.; et al. The Sequences of 1504 Mutants in the Model Rice Variety Kitaake Facilitate Rapid Functional Genomic Studies. Plant Cell 2017, 29, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Kamiya, T.; Shigenobu, S.; Yamaguchi, K.; Yamada, M.; Hasebe, M.; Fujiwara, T.; Sawa, S. Identification of an EMS-induced causal mutation in a gene required for boron-mediated root development by low-coverage genome re-sequencing in Arabidopsis. Plant Signal. Behav. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhen, L.; Liu, Z.; Guo, Y.; Qiu, L.J. Next-Generation Sequencing from Bulked-Segregant Analysis Accelerates the Simultaneous Identification of Two Qualitative Genes in Soybean. Front. Plant Sci. 2017, 8, 919. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chern, M.; Jain, R.; Martin, J.; Schackwitz, W.; Jiang, L.; Vega-Sánchez, M.; Lipzen, A.; Barry, K.; Schmutz, J. Genome-Wide Sequencing of 41 Rice (Oryza sativa L.) Mutated Lines Reveals Diverse Mutations Induced by Fast-Neutron Irradiation. Mol. Plant 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Huang, J.; Fu, H.; Zhou, L.; Furusawa, Y.; Shu, Q. Mutagenic effect of three ion beams on rice and identification of heritable mutations by whole genome sequencing. Plants 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Kazama, Y.; Ishii, K.; Hirano, T.; Wakana, T.; Yamada, M.; Ohbu, S.; Abe, T. Different mutational function of low-and high-linear energy transfer heavy-ion irradiation demonstrated by whole-genome resequencing of Arabidopsis mutants. Plant J. 2017, 92, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.P.K.; Ung, Y.C.; Hussein, S.; Harun, A.R.; Yoshihiro, H. Morphological and biochemical responses of Oryza sativa L. (cultivar MR219) to ion beam irradiation. J. Zhejiang Univ. Sci. B 2013, 14, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G.; O’Neill, P.; Stewart, R.D. Induction and Repair of Clustered DNA Lesions: What Do We Know So Far? Radiat. Res. 2013, 180, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Z.; Liu, Q.; Yang, G.; Zhou, L.; Li, W.; Wang, H.; Chen, Z.; Guo, T. Time Course Analysis of Genome-Wide Identification of Mutations Induced by and Genes Expressed in Response to Carbon Ion Beam Irradiation in Rice. Genes 2021, 12, 1391. [Google Scholar] [CrossRef] [PubMed]
- Ryouhei, Y.; Shigeki, N.; Yoshihiro, H.; Issay, N.; Jun, H.; Sakamoto, A.N. Mutational effects of γ-rays and carbon ion beams on Arabidopsis seedlings. J. Radiat. Res. 2013, 6, 1050–1056. [Google Scholar]
- Benzer, S. On the Topography of the Genetic Fine Structure. Proc. Natl. Acad. Sci. USA 1961, 47, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Kapoor, S.; Sugita, M.; Sugiura, M. Transcript analysis of the tobacco plastid operon rps2/atpl/H/F/A reveals the existence of a nonconsensus type II (NCII) promoter upstream of the atpl coding sequence. Mol. Gen. Genet. 1998, 257, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Arlt, M.F.; Durkin, S.G.; Ragland, R.L.; Glover, T.W. Common fragile sites as targets for chromosome rearrangements. DNA Repair 2006, 5, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H.; Wang, K.; Li, M. Hakonarson HANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]







| Type | Line | Sequenced Generation | HQ_Clean_ Data(bp) | Theoretical Genome Coverage | Genome Coverage of 10× Sequencing Depth (%) | Genome Coverage of 20× Sequencing Depth (%) | Genome Coverage of 30× Sequencing Depth (%) |
|---|---|---|---|---|---|---|---|
| WT | H492 | M6 | 16.0 | 42.9 | 93.79 | 90.22 | 80.01 |
| Mutant | H494 | M6 | 16.2 | 43.5 | 88.05 | 77.08 | 62.57 |
| Mutant | H495 | M6 | 16.2 | 43.3 | 88.72 | 77.47 | 62.03 |
| Mutant | H496 | M6 | 16.4 | 44.1 | 89.67 | 79.73 | 65.36 |
| WT | H499 | M6 | 13.6 | 36.5 | 79.55 | 64.22 | 45.77 |
| Mutant | H512 | M6 | 14.2 | 38.0 | 80.63 | 66.28 | 48.47 |
| WT | H574 | M6 | 15.6 | 41.9 | 85.99 | 79.55 | 64.71 |
| Mutant | H579 | M6 | 15.9 | 42.7 | 86.62 | 79.67 | 64.11 |
| Mutant | H580 | M6 | 16.9 | 45.3 | 86.95 | 80.7 | 67.93 |
| Mutant | H592 | M6 | 16.2 | 43.4 | 86.38 | 79.72 | 65.78 |
| Mutant | H593 | M6 | 16.2 | 43.4 | 86.51 | 80.5 | 66.75 |
| Average | 15.8 | 42.3 | 86.6 | 77.7 | 63.0 |
| WT | Mutant | Total | SBSs | InDels | The Estimated Mutations in M1 | Mutation Rate in M1 |
|---|---|---|---|---|---|---|
| H492 | H494 | 2455 | 2101 | 354 | 4910 | 12.3 × 10−5 |
| H495 | 145 | 110 | 35 | 290 | ||
| H496 | 66,813 | 59,315 | 7498 | 133,626 | ||
| H499 | H512 | 21,101 | 18,860 | 2241 | 42,202 | 11.3 × 10−5 |
| H574 | H579 | 8503 | 7257 | 1246 | 17,006 | 6.0 × 10−5 |
| H580 | 6196 | 5528 | 668 | 12,392 | ||
| H592 | 23,689 | 20,548 | 3141 | 47,378 | ||
| H593 | 6633 | 5860 | 773 | 13,266 | ||
| Average | 16,942 | 14,947 | 1995 | 33,884 | 9.8 × 10−5 |
| Category | Mutant | Synonymous Mutation | Non-Synonymous Mutation | Nonsense Mutation | Frameshift Mutation |
|---|---|---|---|---|---|
| MP1 | H494 | 63 | 62 | 1 | 3 |
| H495 | 0 | 2 | 1 | 0 | |
| H496 | 1411 | 1728 | 39 | 75 | |
| MP2 | H512 | 328 | 465 | 10 | 22 |
| MP3 | H579 | 327 | 302 | 11 | 15 |
| H580 | 105 | 130 | 3 | 7 | |
| H592 | 593 | 496 | 7 | 20 | |
| H593 | 123 | 116 | 3 | 7 | |
| Total | 2950 | 3301 | 75 | 149 | |
| Average | 368.8 | 412.6 | 9.4 | 18.6 |
| Mutant | Gene ID | Whether in HF Mutation Region | Gene Description | Possibly Affected Phenotype | Cloned or Not |
|---|---|---|---|---|---|
| H494 | Os06g0247500 | No | Pyrophosphate-fructose 6-phosphate 1-phosphotransferase | Plant height, grain shape | Yes |
| H495 | Os02g0278400 | No | Cytochrome P450 | Plant height, grain shape | No |
| H496 | Os05g0374200 | No | Cytokinin oxidase/dehydrogenase 9 | Plant height, grain shape, setting percentage | Yes |
| H496 | Os01g0826400 | No | WRKY transcription factor | Plant height, grain shape | Yes |
| H496 | Os07g0214300 | No | α-amylase/trypsin inhibitor | Grain shape | Yes |
| H496 | Os07g0235800 | No | APETALA2-like transcription factor | Grain shape | Yes |
| H496 | Os07g0583700 | No | WRKY transcription factor 78 | Plant height, grain shape | Yes |
| H496 | Os12g0428600 | Yes | HECT-domain E3 ubiquitin ligase | Plant height, grain shape | Yes |
| H496 | Os12g0496900 | No | GRF-INTERACTING FACTOR 2 | Plant height, grain shape | No |
| H496 | Os12g0552600 | No | Grain Weight (qTGW12a) | Grain shape | Yes |
| H512 | Os01g0972800 | No | WRKY transcription factor | Plant height, grain length | No |
| H512 | Os03g0430000 | Yes | defective embryo sac1 | Setting percentage | Yes |
| H512 | Os05g0547850 | No | Programmed cell death 5 | Plant height, grain shape | Yes |
| H580 | Os04g0396500 | No | lax panicle2 | Panicle type | Yes |
| H592 | Os03g0267800 | No | Ubiquitin-interacting motif-containing ubiquitin receptor | Grain shape | Yes |
| H592 | Os03g0274800 | Yes | Receptor-like cytoplasmic kinase | Plant archetechture, setting percentage | Yes |
| H592 | Os07g0192300 | Yes | NARROW LEAF 11 | Plant archetechture, panicle type | Yes |
| H592 | Os07g0232100 | Yes | B3 domain transcription factor | Panicle type | Yes |
| H592 | Os07g0233300 | Yes | Alfin-like gene | Grain length | Yes |
| H592 | Os07g0235800 | Yes | APETALA2-like transcription factor | Grain shape | Yes |
| H592 | Os09g0281900 | No | Mediator subunit gene | Plant height, grain shape, setting percentage | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Zhang, Y.; Huang, M.; Guo, T.; Yang, G. Genome-Wide Profile of Mutations Induced by Carbon Ion Beam Irradiation of Dehulled Rice Seeds. Int. J. Mol. Sci. 2024, 25, 5195. https://doi.org/10.3390/ijms25105195
Ling Y, Zhang Y, Huang M, Guo T, Yang G. Genome-Wide Profile of Mutations Induced by Carbon Ion Beam Irradiation of Dehulled Rice Seeds. International Journal of Molecular Sciences. 2024; 25(10):5195. https://doi.org/10.3390/ijms25105195
Chicago/Turabian StyleLing, Ying, Yuming Zhang, Ming Huang, Tao Guo, and Guili Yang. 2024. "Genome-Wide Profile of Mutations Induced by Carbon Ion Beam Irradiation of Dehulled Rice Seeds" International Journal of Molecular Sciences 25, no. 10: 5195. https://doi.org/10.3390/ijms25105195





