The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature
Abstract
:1. Introduction
2. Methodology Employed for Study Selection
3. Implications of One-Carbon Metabolism in Medically Assisted Reproduction
3.1. Outline of One Carbon Metabolism
3.2. One-Carbon Metabolism, Infertility, and Medically Assisted Reproduction
4. One-Carbon Metabolism and Medically Assisted Reproduction: Clinical Implications
4.1. Clinical Implications of Zinc in Medically Assisted Reproduction
4.2. Clinical Implications of Folate in Medically Assisted Reproduction
4.3. Clinical Implications of Vitamin B12 in Medically Assisted Reproduction
4.4. Clinical Implications of Choline in Medically Assisted Reproduction
4.5. Clinical Implications of Betaine in Medically Assisted Reproduction
4.6. Clinical Implications of Homocysteine in Medically Assisted Reproduction
4.7. The Role of Metabolomics in the Era of Personalized and Precision Medicine in Assisted Reproduction
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anifandis, G.; Messini, C.I.; Dafopoulos, K.; Messinis, I.E. Genes and Conditions Controlling Mammalian Pre- and Post-Implantation Embryo Development. Curr. Genom. 2015, 16, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA Methylation Patterns and Epigenetic Memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA Methylation in the Mammalian Life Cycle: Building and Breaking Epigenetic Barriers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef]
- Mitchell, L.E. Maternal Effect Genes: Update and Review of Evidence for a Link with Birth Defects. HGG Adv. 2021, 3, 100067. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic Programs in Human and Mouse Early Embryos Revealed by Single-Cell RNA Sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef]
- Rubini, E.; Baijens, I.M.M.; Horánszky, A.; Schoenmakers, S.; Sinclair, K.D.; Zana, M.; Dinnyés, A.; Steegers-Theunissen, R.P.M.; Rousian, M. Maternal One-Carbon Metabolism during the Periconceptional Period and Human Foetal Brain Growth: A Systematic Review. Genes 2021, 12, 1634. [Google Scholar] [CrossRef]
- Clare, C.E.; Pestinger, V.; Kwong, W.Y.; Tutt, D.A.R.; Xu, J.; Byrne, H.M.; Barrett, D.A.; Emes, R.D.; Sinclair, K.D. Interspecific Variation in One-Carbon Metabolism within the Ovarian Follicle, Oocyte, and Preimplantation Embryo: Consequences for Epigenetic Programming of DNA Methylation. Int. J. Mol. Sci. 2021, 22, 1838. [Google Scholar] [CrossRef]
- Gurwara, S.; Ajami, N.J.; Jang, A.; Hessel, F.C.; Chen, L.; Plew, S.; Wang, Z.; Graham, D.Y.; Hair, C.; White, D.L.; et al. Dietary Nutrients Involved in One-Carbon Metabolism and Colonic Mucosa-Associated Gut Microbiome in Individuals with an Endoscopically Normal Colon. Nutrients 2019, 11, 613. [Google Scholar] [CrossRef]
- Dayon, L.; Guiraud, S.P.; Corthésy, J.; Da Silva, L.; Migliavacca, E.; Tautvydaitė, D.; Oikonomidi, A.; Moullet, B.; Henry, H.; Métairon, S.; et al. One-Carbon Metabolism, Cognitive Impairment and CSF Measures of Alzheimer Pathology: Homocysteine and Beyond. Alzheimers Res. Ther. 2017, 9, 43. [Google Scholar] [CrossRef]
- Danchin, A. Zinc, an Unexpected Integrator of Metabolism? Microb. Biotechnol. 2020, 13, 895–898. [Google Scholar] [CrossRef]
- Danchin, A.; Sekowska, A.; You, C. One-Carbon Metabolism, Folate, Zinc and Translation. Microb. Biotechnol. 2020, 13, 899–925. [Google Scholar] [CrossRef]
- Azimi, Z.; Isa, M.R.; Khan, J.; Wang, S.M.; Ismail, Z. Association of Zinc Level with DNA Methylation and Its Consequences: A Systematic Review. Heliyon 2022, 8, e10815. [Google Scholar] [CrossRef] [PubMed]
- Dattilo, M.; Cornet, D.; Amar, E.; Cohen, M.; Menezo, Y. The Importance of the One Carbon Cycle Nutritional Support in Human Male Fertility: A Preliminary Clinical Report. Reprod. Biol. Endocrinol. 2014, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-Carbon Metabolism in Cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.M.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The Periconceptional Period, Reproduction and Long-Term Health of Offspring: The Importance of One-Carbon Metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The Origins of the Developmental Origins Theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Cai, S.; Quan, S.; Yang, G.; Chen, M.; Ye, Q.; Wang, G.; Yu, H.; Wang, Y.; Qiao, S.; Zeng, X. Nutritional Status Impacts Epigenetic Regulation in Early Embryo Development: A Scoping Review. Adv. Nutr. 2021, 12, 1877–1892. [Google Scholar] [CrossRef]
- Wiklund, P.; Karhunen, V.; Richmond, R.C.; Parmar, P.; Rodriguez, A.; De Silva, M.; Wielscher, M.; Rezwan, F.I.; Richardson, T.G.; Veijola, J.; et al. DNA Methylation Links Prenatal Smoking Exposure to Later Life Health Outcomes in Offspring. Clin. Epigenetics 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Niemitz, E.L.; Feinberg, A.P. Association of in Vitro Fertilization with Beckwith-Wiedemann Syndrome and Epigenetic Alterations of LIT1 and H19. Am. J. Hum. Genet. 2003, 72, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Akamine, K.; Mekaru, K.; Gibo, K.; Nagata, C.; Nakamura, R.; Oishi, S.; Miyagi, M.; Heshiki, C.; Aoki, Y. Impact of the One-Carbon Metabolism on Oocyte Maturation, Fertilization, Embryo Quality, and Subsequent Pregnancy. Reprod. Med. Biol. 2021, 20, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Koyama, H.; Sugimoto, M.; Kume, S. Roles of One-Carbon Metabolism in Preimplantation Period: Effects on Short-Term Development and Long-Term Programming. J. Reprod. Dev. 2012, 58, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Jaiswal, D. One-Carbon Metabolism, Spermatogenesis, and Male Infertility. Reprod. Sci. 2013, 20, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P.; McCallum, H.; McBain, H.; Andrews, K.; Duthie, S.; McNeill, G.; Templeton, A.; Haites, N.; Campbell, D.; Bhattacharya, S. Effect of B Vitamins and Genetics on Success of In-Vitro Fertilisation: Prospective Cohort Study. Lancet 2006, 367, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Martel, J.; Karahan, G.; Angle, C.; Behan, N.A.; Chan, D.; MacFarlane, A.J.; Trasler, J.M. Moderate Maternal Folic Acid Supplementation Ameliorates Adverse Embryonic and Epigenetic Outcomes Associated with Assisted Reproduction in a Mouse Model. Hum. Reprod. 2019, 34, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.; Steegers-Theunissen, R.P.M.; Willemsen, S.P.; Schoenmakers, S. Paternal Folate Status and Sperm Quality, Pregnancy Outcomes, and Epigenetics: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2020, 64, e1900696. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Rousian, M.; Koning, A.H.J.; Willemsen, S.P.; Cetin, I.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Periconceptional Maternal Biomarkers of One-Carbon Metabolism and Embryonic Growth Trajectories: The Rotterdam Periconceptional Cohort (Predict Study). Fertil. Steril. 2017, 107, 691–698.e1. [Google Scholar] [CrossRef]
- O’Neill, R.J.; Vrana, P.B.; Rosenfeld, C.S. Maternal Methyl Supplemented Diets and Effects on Offspring Health. Front. Genet. 2014, 5, 289. [Google Scholar] [CrossRef]
- Anckaert, E.; Romero, S.; Adriaenssens, T.; Smitz, J. Effects of Low Methyl Donor Levels in Culture Medium during Mouse Follicle Culture on Oocyte Imprinting Establishment. Biol. Reprod. 2010, 83, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jing, X.; Wu, X.; Yan, M.; Li, Q.; Shen, Y.; Wang, Z. DNA Methylation in Spermatogenesis and Male Infertility. Exp. Ther. Med. 2016, 12, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Håberg, S.E.; Page, C.M.; Lee, Y.; Nustad, H.E.; Magnus, M.C.; Haftorn, K.L.; Carlsen, E.Ø.; Denault, W.R.P.; Bohlin, J.; Jugessur, A.; et al. DNA Methylation in Newborns Conceived by Assisted Reproductive Technology. Nat. Commun. 2022, 13, 1896. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J. One-Carbon Metabolism-Genome Interactions in Folate-Associated Pathologies. J. Nutr. 2009, 139, 2402–2405. [Google Scholar] [CrossRef] [PubMed]
- Stover, P.J.; Field, M.S. Trafficking of Intracellular Folates. Adv. Nutr. 2011, 2, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lucock, M. Folic Acid: Nutritional Biochemistry, Molecular Biology, and Role in Disease Processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Bozack, A.K.; Gamble, M.V. Nutritional Influences on One-Carbon Metabolism: Effects on Arsenic Methylation and Toxicity. Annu. Rev. Nutr. 2018, 38, 401–429. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B. Biomarkers of Nutrient Exposure and Status in One-Carbon (Methyl) Metabolism. J. Nutr. 2003, 133 (Suppl. S3), 941S–947S. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cantley, L.C. Toward a Better Understanding of Folate Metabolism in Health and Disease. J. Exp. Med. 2019, 216, 253–266. [Google Scholar] [CrossRef]
- Annibal, A.; Tharyan, R.G.; Schonewolff, M.F.; Tam, H.; Latza, C.; Auler, M.M.K.; Grönke, S.; Partridge, L.; Antebi, A. Regulation of the One Carbon Folate Cycle as a Shared Metabolic Signature of Longevity. Nat. Commun. 2021, 12, 3486. [Google Scholar] [CrossRef]
- Garratt, L.C.; Ortori, C.A.; Tucker, G.A.; Sablitzky, F.; Bennett, M.J.; Barrett, D.A. Comprehensive Metabolic Profiling of Mono- and Polyglutamated Folates and Their Precursors in Plant and Animal Tissue Using Liquid Chromatography/Negative Ion Electrospray Ionisation Tandem Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2005, 19, 2390–2398. [Google Scholar] [CrossRef]
- Engevik, M.A.; Morra, C.N.; Röth, D.; Engevik, K.; Spinler, J.K.; Devaraj, S.; Crawford, S.E.; Estes, M.K.; Kalkum, M.; Versalovic, J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019, 10, 2305. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic Acid versus 5- Methyl Tetrahydrofolate Supplementation in Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Takata, Y.; Huang, Y.; Komoto, J.; Yamada, T.; Konishi, K.; Ogawa, H.; Gomi, T.; Fujioka, M.; Takusagawa, F. Catalytic Mechanism of Glycine N-Methyltransferase. Biochemistry 2003, 42, 8394–8402. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur Amino Acid Metabolism: Pathways for Production and Removal of Homocysteine and Cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sinclair, K.D. One-Carbon Metabolism and Epigenetic Regulation of Embryo Development. Reprod. Fertil. Dev. 2015, 27, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.G.; Petrova, B.; Kanarek, N. Notes from the 2022 Folate, Vitamin B12, and One-Carbon Metabolism Conference. Metabolites 2023, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Shane, B. Folate and Vitamin B12 Metabolism: Overview and Interaction with Riboflavin, Vitamin B6, and Polymorphisms. Food Nutr. Bull. 2008, 29, S5–S16; discussion S17–S19. [Google Scholar] [CrossRef]
- Tjong, E.; Dimri, M.; Mohiuddin, S.S. Biochemistry, Tetrahydrofolate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hitchings, G.H. Functions of Tetrahydrofolate and the Role of Dihydrofolate Reductase in Cellular Metabolism. In Inhibition of Folate Metabolism in Chemotherapy: The Origins and Uses of Co-Trimoxazole; Hitchings, G.H., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1983; pp. 11–23. ISBN 978-3-642-81890-5. [Google Scholar]
- Mentch, S.J.; Locasale, J.W. One-Carbon Metabolism and Epigenetics: Understanding the Specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Shuvalov, O.; Petukhov, A.; Daks, A.; Fedorova, O.; Vasileva, E.; Barlev, N.A. One-Carbon Metabolism and Nucleotide Biosynthesis as Attractive Targets for Anticancer Therapy. Oncotarget 2017, 8, 23955–23977. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Tamura, T. Folate-Dependent Purine Nucleotide Biosynthesis in Humans1. Adv. Nutr. 2015, 6, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, R.; Gao, X.; Lin, Z.; Tang, H.; Cui, H.; Zhao, E. Targeting Serine-Glycine-One-Carbon Metabolism as a Vulnerability in Cancers. Biomark. Res. 2023, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Roje, S. S-Adenosyl-L-Methionine: Beyond the Universal Methyl Group Donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The Impact of Metabolism on DNA Methylation. Hum. Mol. Genet. 2005, 14, R139–R147. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Wang, J.C.; Melnyk, S.; Pogribny, I.P.; Jernigan, S.; Collins, M.D.; Santos-Guzman, J.; Swendseid, M.E.; Cogger, E.A.; James, S.J. Intracellular S-Adenosylhomocysteine Concentrations Predict Global DNA Hypomethylation in Tissues of Methyl-Deficient Cystathionine Beta-Synthase Heterozygous Mice. J. Nutr. 2001, 131, 2811–2818. [Google Scholar] [CrossRef]
- Sun, Y.; Locasale, J.W. Rethinking the Bioavailability and Cellular Transport Properties of S-Adenosylmethionine. Cell Stress. 2022, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, A.Y.; Mariasina, S.S.; Sergiev, P.V.; Polshakov, V.I. Analogs of S-Adenosyl-L-Methionine in Studies of Methyltransferases. Mol. Biol. 2022, 56, 229–250. [Google Scholar] [CrossRef]
- Serefidou, M.; Venkatasubramani, A.V.; Imhof, A. The Impact of One Carbon Metabolism on Histone Methylation. Front. Genet. 2019, 10, 764. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef]
- Fischer, T.R.; Meidner, L.; Schwickert, M.; Weber, M.; Zimmermann, R.A.; Kersten, C.; Schirmeister, T.; Helm, M. Chemical Biology and Medicinal Chemistry of RNA Methyltransferases. Nucleic Acids Res. 2022, 50, 4216–4245. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Cho, Y.; Bae, G.-U.; Kim, S.-N.; Kim, Y.K. Protein Arginine Methyltransferases: Promising Targets for Cancer Therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef] [PubMed]
- Husmann, D.; Gozani, O. Histone Lysine Methyltransferases in Biology and Disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Zabala-Letona, A.; Arruabarrena-Aristorena, A.; Fernandez-Ruiz, S.; Viera, C.; Carlevaris, O.; Ercilla, A.; Mendizabal, I.; Martin, T.; Macchia, A.; Camacho, L.; et al. PI3K-Regulated Glycine N-Methyltransferase Is Required for the Development of Prostate Cancer. Oncogenesis 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, L.; Kaiser, P. Sensing and Signaling of Methionine Metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, Q.; Lee, C.; Wang, S.; Pelleymounter, L.L.; Moon, I.; Eckloff, B.W.; Wieben, E.D.; Schaid, D.J.; Yee, V.; et al. Human Betaine-Homocysteine Methyltransferase (BHMT) and BHMT2: Common Gene Sequence Variation and Functional Characterization. Mol. Genet. Metab. 2008, 94, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.P.M.; Ueland, P.M.; Clarke, R.; Blom, H.J.; Hoefnagels, W.H.L.; van Staveren, W.A.; de Groot, L.C.P.G.M. The Association of Betaine, Homocysteine and Related Metabolites with Cognitive Function in Dutch Elderly People. Br. J. Nutr. 2007, 98, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Giovannucci, E.L.; Hankinson, S.E.; Zeisel, S.H.; Dougherty, L.W.; Willett, W.C.; Rimm, E.B. The Association between Betaine and Choline Intakes and the Plasma Concentrations of Homocysteine in Women. Am. J. Clin. Nutr. 2007, 86, 1073–1081. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Is There an Effect of Methyl Donor Nutrient Supplementation on Metabolic Syndrome in Humans? Med. Sci. 2020, 8, 2. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the Transsulfuration Pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef]
- McBean, G.J.; Aslan, M.; Griffiths, H.R.; Torrão, R.C. Thiol Redox Homeostasis in Neurodegenerative Disease. Redox Biol. 2015, 5, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524. [Google Scholar] [CrossRef]
- Surai, P.F.; Earle-Payne, K.; Kidd, M.T. Taurine as a Natural Antioxidant: From Direct Antioxidant Effects to Protective Action in Various Toxicological Models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The Antioxidant Glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Storch, K.J.; Wagner, D.A.; Burke, J.F.; Young, V.R. [1-13C; Methyl-2H3]Methionine Kinetics in Humans: Methionine Conservation and Cystine Sparing. Am. J. Physiol. 1990, 258, E790–E798. [Google Scholar] [CrossRef]
- Selhub, J. Homocysteine Metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Thakur, J.; Suyal, S.; Oniel, R.; Chakraborty, R.; Pradhan, S.; Sharma, M.; Sengupta, S.; Laxman, S.; Masakapalli, S.K.; et al. Allosteric Inhibition of MTHFR Prevents Futile SAM Cycling and Maintains Nucleotide Pools in One-Carbon Metabolism. J. Biol. Chem. 2020, 295, 16037–16057. [Google Scholar] [CrossRef]
- Bravo, A.C.; Aguilera, M.N.L.; Marziali, N.R.; Moritz, L.; Wingert, V.; Klotz, K.; Schumann, A.; Grünert, S.C.; Spiekerkoetter, U.; Berger, U.; et al. Analysis of S-Adenosylmethionine and S-Adenosylhomocysteine: Method Optimisation and Profiling in Healthy Adults upon Short-Term Dietary Intervention. Metabolites 2022, 12, 373. [Google Scholar] [CrossRef]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the Growing Science on Its Benefits for Moms and Babies. Nutrients 2019, 11, E1823. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, Z.; Zhou, J.; Li, W.; Dong, Y.; Qian, Y.; Wei, P.; Wu, M. Associations of One-Carbon Metabolism-Related Gene Polymorphisms with Breast Cancer Risk Are Modulated by Diet, Being Higher When Adherence to the Mediterranean Dietary Pattern Is Low. Breast Cancer Res. Treat. 2021, 187, 793–804. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 1998; ISBN 978-0-309-06411-8. [Google Scholar]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Rech, L.; Wu, Y.; Goltz, D.; Taylor, C.G.; House, J.D. Effects of Zinc Deficiency and Zinc Supplementation on Homocysteine Levels and Related Enzyme Expression in Rats. J. Trace Elem. Med. Biol. 2015, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Resseguie, M.; Song, J.; Niculescu, M.D.; da Costa, K.-A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-Methyltransferase (PEMT) Gene Expression Is Induced by Estrogen in Human and Mouse Primary Hepatocytes. FASEB J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.M.; daCosta, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.-S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and Menopausal Status Influence Human Dietary Requirements for the Nutrient Choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.M.; Ruiz-Echevarría, M.J. One-Carbon Metabolism in Prostate Cancer: The Role of Androgen Signaling. Int. J. Mol. Sci. 2016, 17, 1208. [Google Scholar] [CrossRef]
- Kim, R.; Nijhout, H.F.; Reed, M.C. One-Carbon Metabolism during the Menstrual Cycle and Pregnancy. PLoS Comput. Biol. 2021, 17, e1009708. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal Nutrient Supplementation Counteracts Bisphenol A-Induced DNA Hypomethylation in Early Development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Martin, E.; Fry, R.C. Effects of Prenatal Exposure to Endocrine Disruptors and Toxic Metals on the Fetal Epigenome. Epigenomics 2017, 9, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Liu, X.; Ahsan, H.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Genomic Methylation of Peripheral Blood Leukocyte DNA: Influences of Arsenic and Folate in Bangladeshi Adults. Am. J. Clin. Nutr. 2007, 86, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.-H.; Souter, I.; Williams, P.L.; Calafat, A.M.; Hauser, R.; Chavarro, J.E.; EARTH Study team. Dietary Folate Intake and Modification of the Association of Urinary Bisphenol a Concentrations with In Vitro Fertilization Outcomes among Women from a Fertility Clinic. Reprod. Toxicol. 2016, 65, 104–112. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Hauser, R.; Gaskins, A.J. Effects of Bisphenol A on Male and Couple Reproductive Health: A Review. Fertil. Steril. 2016, 106, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Schaevitz, L.; Berger-Sweeney, J.; Ricceri, L. One-Carbon Metabolism in Neurodevelopmental Disorders: Using Broad-Based Nutraceutics to Treat Cognitive Deficits in Complex Spectrum Disorders. Neurosci. Biobehav. Rev. 2014, 46 Pt 2, 270–284. [Google Scholar] [CrossRef]
- Zahed, H.; Johansson, M.; Ueland, P.M.; Midttun, Ø.; Milne, R.L.; Giles, G.G.; Manjer, J.; Sandsveden, M.; Langhammer, A.; Sørgjerd, E.P.; et al. Epidemiology of 40 Blood Biomarkers of One-Carbon Metabolism, Vitamin Status, Inflammation, and Renal and Endothelial Function among Cancer-Free Older Adults. Sci. Rep. 2021, 11, 13805. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Simile, M.M.; Ladu, S.; Pellegrino, R.; De Murtas, V.; Pinna, F.; Tomasi, M.L.; Frau, M.; Virdis, P.; De Miglio, M.R.; et al. Altered Methionine Metabolism and Global DNA Methylation in Liver Cancer: Relationship with Genomic Instability and Prognosis. Int. J. Cancer 2007, 121, 2410–2420. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Shawkat Ahmed, H.; Noori, S.H. The Importance of Serum Homocysteine as a Biomarker in Diabetic and Obese COVID-19 Patients. Cell Mol. Biol. 2023, 69, 52–59. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review12345. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal Folate, One-Carbon Metabolism and Pregnancy Outcomes. Matern. Child. Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Wood, C.; Mina, T.H.; Webster, C.; Goljan, I.; Weldeselassie, Y.; Reynolds, R.M.; Saravanan, P. Vitamin B12 Deficiency and Altered One-Carbon Metabolites in Early Pregnancy Is Associated with Maternal Obesity and Dyslipidaemia. Sci. Rep. 2020, 10, 11066. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility Prevalence and the Methods of Estimation from 1990 to 2021: A Systematic Review and Meta-Analysis. Hum Reprod Open 2022, 2022, hoac051. [Google Scholar] [CrossRef] [PubMed]
- Njagi, P.; Groot, W.; Arsenijevic, J.; Dyer, S.; Mburu, G.; Kiarie, J. Financial Costs of Assisted Reproductive Technology for Patients in Low- and Middle-Income Countries: A Systematic Review. Human. Reprod. Open 2023, 2023, hoad007. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Raperport, C.; Desai, J.; Qureshi, D.; Rustin, E.; Balaji, A.; Chronopoulou, E.; Homburg, R.; Khan, K.S.; Bhide, P. The Definition of Unexplained Infertility: A Systematic Review. BJOG 2023, 131, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.S.; Cruz Ithier, M.A.; Rosen, T.; Ashkinadze, E. Advanced Paternal Age, Infertility, and Reproductive Risks: A Review of the Literature. Prenat. Diagn. 2019, 39, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, I.; Verbiest, S.; Tydén, T. Knowledge about the Impact of Age on Fertility: A Brief Review. Ups. J. Med. Sci. 2020, 125, 167–174. [Google Scholar] [CrossRef]
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the Aging Male: A Systematic Review. Fertil. Steril. 2022, 118, 1022–1034. [Google Scholar] [CrossRef]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.-M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental Factors in Declining Human Fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Emokpae, M.A.; Brown, S.I. Effects of Lifestyle Factors on Fertility: Practical Recommendations for Modification. Reprod. Fertil. 2021, 2, R13–R26. [Google Scholar] [CrossRef]
- Pelikh, A.; Smith, K.R.; Myrskylä, M.; Goisis, A. Medically Assisted Reproduction Treatment Types and Birth Outcomes: A Between-Family and Within-Family Analysis. Obstet. Gynecol. 2022, 139, 211–222. [Google Scholar] [CrossRef]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L.R. Sperm Selection in Natural Conception: What Can We Learn from Mother Nature to Improve Assisted Reproduction Outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef]
- Sciorio, R.; El Hajj, N. Epigenetic Risks of Medically Assisted Reproduction. J. Clin. Med. 2022, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Loft, A.; Romundstad, L.B.; Wennerholm, U.-B.; Söderström-Anttila, V.; Bergh, C.; Aittomäki, K. Epigenetics and Assisted Reproductive Technologies. Acta Obstet. Gynecol. Scand. 2016, 95, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Reyes Palomares, A.; Rodriguez-Wallberg, K.A. Update on the Epigenomic Implication of Embryo Cryopreservation Methods Applied in Assisted Reproductive Technologies with Potential Long-Term Health Effects. Front. Cell Dev. Biol. 2022, 10, 881550. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Tang, F.; Yan, L.; Qiao, J. Epigenetic Regulation and Risk Factors During the Development of Human Gametes and Early Embryos. Annu. Rev. Genom. Hum. Genet. 2019, 20, 21–40. [Google Scholar] [CrossRef]
- Ghosh, J.; Coutifaris, C.; Sapienza, C.; Mainigi, M. Global DNA Methylation Levels Are Altered by Modifiable Clinical Manipulations in Assisted Reproductive Technologies. Clin. Epigenetics 2017, 9, 14. [Google Scholar] [CrossRef]
- Berntsen, S.; Söderström-Anttila, V.; Wennerholm, U.-B.; Laivuori, H.; Loft, A.; Oldereid, N.B.; Romundstad, L.B.; Bergh, C.; Pinborg, A. The Health of Children Conceived by ART: “The Chicken or the Egg?”. Hum. Reprod. Update 2019, 25, 137–158. [Google Scholar] [CrossRef]
- Schiuma, N.; Costantino, A.; Bartolotti, T.; Dattilo, M.; Bini, V.; Aglietti, M.C.; Renga, M.; Favilli, A.; Falorni, A.; Gerli, S. Micronutrients in Support to the One Carbon Cycle for the Modulation of Blood Fasting Homocysteine in PCOS Women. J. Endocrinol. Investig. 2020, 43, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, J.; He, B.; Jia, Y.; Niu, Y.; Wang, C.; Zhao, R. Abnormally Activated One-Carbon Metabolic Pathway Is Associated with mtDNA Hypermethylation and Mitochondrial Malfunction in the Oocytes of Polycystic Gilt Ovaries. Sci. Rep. 2016, 6, 19436. [Google Scholar] [CrossRef] [PubMed]
- Sibuh, B.Z.; Quazi, S.; Panday, H.; Parashar, R.; Jha, N.K.; Mathur, R.; Jha, S.K.; Taneja, P.; Jha, A.K. The Emerging Role of Epigenetics in Metabolism and Endocrinology. Biology 2023, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, F.M.; Cimadomo, D.; Vaiarelli, A.; Fabozzi, G.; Venturella, R.; Maggiulli, R.; Mazzilli, R.; Ferrero, S.; Palagiano, A.; Rienzi, L. Advanced Maternal Age in IVF: Still a Challenge? The Present and the Future of Its Treatment. Front. Endocrinol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced Maternal Age and Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef] [PubMed]
- Machado-Gédéon, A.; Badeghiesh, A.; Baghlaf, H.; Dahan, M.H. Adverse Pregnancy, Delivery and Neonatal Outcomes across Different Advanced Maternal Ages: A Population-Based Retrospective Cohort Study. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2023, 17, 100180. [Google Scholar] [CrossRef] [PubMed]
- Bebbere, D.; Coticchio, G.; Borini, A.; Ledda, S. Oocyte Aging: Looking beyond Chromosome Segregation Errors. J. Assist. Reprod. Genet. 2022, 39, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Fernandez, J.E.; Loke, Y.J.; Bass-Stringer, S.; Gao, F.; Xia, Y.; Wu, H.; Lu, H.; Liu, Y.; Wang, J.; Spector, T.D.; et al. DNA Methylation Changes at Infertility Genes in Newborn Twins Conceived by in Vitro Fertilisation. Genome Med. 2017, 9, 28. [Google Scholar] [CrossRef]
- Yue, M.; Fu, X.; Zhou, G.; Hou, Y.; Du, M.; Wang, L.; Zhu, S. Abnormal DNA Methylation in Oocytes Could Be Associated with a Decrease in Reproductive Potential in Old Mice. J. Assist. Reprod. Genet. 2012, 29, 643–650. [Google Scholar] [CrossRef]
- Guglielmino, M.R.; Santonocito, M.; Vento, M.; Ragusa, M.; Barbagallo, D.; Borzì, P.; Casciano, I.; Banelli, B.; Barbieri, O.; Astigiano, S.; et al. TAp73 Is Downregulated in Oocytes from Women of Advanced Reproductive Age. Cell Cycle 2011, 10, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Tomasini, R.; McKeon, F.D.; Mak, T.W.; Melino, G. The P53 Family: Guardians of Maternal Reproduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-P.; Thurston, A.; Mummery, C.; Ward-van Oostwaard, D.; Priddle, H.; Allegrucci, C.; Denning, C.; Young, L. Gene-Specific Vulnerability to Imprinting Variability in Human Embryonic Stem Cell Lines. Genome Res. 2007, 17, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.A.J.; Schomakers, B.V.; van Weeghel, M.; Wever, E.J.M.; Wüst, R.C.I.; Dijk, F.; Janssens, G.E.; Goddijn, M.; Mastenbroek, S.; Houtkooper, R.H.; et al. Human Ovarian Aging Is Characterized by Oxidative Damage and Mitochondrial Dysfunction. Hum. Reprod. 2023, 38, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-Associated Epigenetic Changes in Mammalian Sperm: Implications for Offspring Health and Development. Hum. Reprod. Update 2022, 29, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.K.; Robaire, B. Advanced Paternal Age and Future Generations. Front. Endocrinol. 2022, 13, 897101. [Google Scholar] [CrossRef]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Sigman, M.; Collura, B.; De Jonge, C.J.; Eisenberg, M.L.; Lamb, D.J.; Mulhall, J.P.; Niederberger, C.; Sandlow, J.I.; Sokol, R.Z.; et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J. Urol. 2021, 205, 36–43. [Google Scholar] [CrossRef]
- Gunes, S.; Hekim, G.N.T.; Arslan, M.A.; Asci, R. Effects of Aging on the Male Reproductive System. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef]
- Paoli, D.; Pecora, G.; Pallotti, F.; Faja, F.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cytological and Molecular Aspects of the Ageing Sperm. Hum. Reprod. 2019, 34, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.A.; Alex, A.; Werlin, L.B.; Marrs, R.P. Age Thresholds for Changes in Semen Parameters in Men. Fertil. Steril. 2013, 100, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, J.M.; Veltman, J.A.; Gilissen, C. De Novo Mutations Reflect Development and Aging of the Human Germline. Trends Genet. 2019, 35, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Djira, G.; Kasperson, K.; Christianson, J. Relationships between the Age of 25,445 Men Attending Infertility Clinics and Sperm Chromatin Structure Assay (SCSA®) Defined Sperm DNA and Chromatin Integrity. Fertil. Steril. 2020, 114, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.; Höffken, V.; Schlatt, S.; Kliesch, S.; Gromoll, J.; Wistuba, J. Ageing in Men with Normal Spermatogenesis Alters Spermatogonial Dynamics and Nuclear Morphology in Sertoli Cells. Andrology 2019, 7, 827–839. [Google Scholar] [CrossRef]
- Yatsenko, A.N.; Turek, P.J. Reproductive Genetics and the Aging Male. J. Assist. Reprod. Genet. 2018, 35, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Ryan, D.P.; Pearson, B.L.; Henzel, K.S.; Neff, F.; Vidal, R.O.; Hennion, M.; Lehmann, I.; Schleif, M.; Schröder, S.; et al. Epigenetic Alterations in Longevity Regulators, Reduced Life Span, and Exacerbated Aging-Related Pathology in Old Father Offspring Mice. Proc. Natl. Acad. Sci. USA 2018, 115, E2348–E2357. [Google Scholar] [CrossRef] [PubMed]
- Tatehana, M.; Kimura, R.; Mochizuki, K.; Inada, H.; Osumi, N. Comprehensive Histochemical Profiles of Histone Modification in Male Germline Cells during Meiosis and Spermiogenesis: Comparison of Young and Aged Testes in Mice. PLoS ONE 2020, 15, e0230930. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bai, D.; Liu, W.; Liu, Y.; Zhang, Y.; Kou, X.; Chen, J.; Wang, H.; Teng, X.; Zuo, J.; et al. Altered Sperm tsRNAs in Aged Male Contribute to Anxiety-like Behavior in Offspring. Aging Cell 2021, 20, e13466. [Google Scholar] [CrossRef]
- Wu, C.; Blondin, P.; Vigneault, C.; Labrecque, R.; Sirard, M.-A. Sperm miRNAs— Potential Mediators of Bull Age and Early Embryo Development. BMC Genom. 2020, 21, 798. [Google Scholar] [CrossRef]
- Khandwala, Y.S.; Baker, V.L.; Shaw, G.M.; Stevenson, D.K.; Lu, Y.; Eisenberg, M.L. Association of Paternal Age with Perinatal Outcomes between 2007 and 2016 in the United States: Population Based Cohort Study. BMJ 2018, 363, k4372. [Google Scholar] [CrossRef]
- Green, R.F.; Devine, O.; Crider, K.S.; Olney, R.S.; Archer, N.; Olshan, A.F.; Shapira, S.K. National Birth Defects Prevention Study Association of Paternal Age and Risk for Major Congenital Anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann. Epidemiol. 2010, 20, 241–249. [Google Scholar] [CrossRef]
- Virdi, S.; McKee, A.M.; Nuthi, M.; Jadavji, N.M. The Role of One-Carbon Metabolism in Healthy Brain Aging. Nutrients 2023, 15, 3891. [Google Scholar] [CrossRef]
- Skoracka, K.; Ratajczak, A.E.; Rychter, A.M.; Dobrowolska, A.; Krela-Kaźmierczak, I. Female Fertility and the Nutritional Approach: The Most Essential Aspects. Adv. Nutr. 2021, 12, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Nema, J.; Joshi, N.; Sundrani, D.; Joshi, S. Influence of Maternal One Carbon Metabolites on Placental Programming and Long Term Health. Placenta 2022, 125, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; Christensen, K.E.; Chan, D.; Leclerc, D.; Landry, M.; Ly, L.; Rozen, R.; Trasler, J. Testicular MTHFR Deficiency May Explain Sperm DNA Hypomethylation Associated with High Dose Folic Acid Supplementation. Hum. Mol. Genet. 2018, 27, 1123–1135. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, Z.; Zhang, L.; Liu, N. MTHFR 677TT Is Associated with Decreased Number of Embryos and Cumulative Live Birth Rate in Patients Undergoing GnRHa Short Protocol: A Retrospective Study. BMC Pregnancy Childbirth 2022, 22, 170. [Google Scholar] [CrossRef]
- Thakur, P.; Bhalerao, A. High Homocysteine Levels During Pregnancy and Its Association With Placenta-Mediated Complications: A Scoping Review. Cureus 2023, 15, e35244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, T.; Ye, L.; Li, G.; Zeng, Y.; Zhang, Y. Prevalent Genotypes of Methylenetetrahydrofolate Reductase (MTHFR) in Recurrent Miscarriage and Recurrent Implantation Failure. J. Assist. Reprod. Genet. 2018, 35, 1437–1442. [Google Scholar] [CrossRef]
- Rah, H.; Jeon, Y.J.; Choi, Y.; Shim, S.H.; Yoon, T.K.; Choi, D.H.; Cha, S.H.; Kim, N.K. Association of Methylenetetrahydrofolate Reductase (MTHFR 677C>T) and Thymidylate Synthase (TSER and TS 1494del6) Polymorphisms with Premature Ovarian Failure in Korean Women. Menopause 2012, 19, 1260–1266. [Google Scholar] [CrossRef]
- Ota, K.; Takahashi, T.; Han, A.; Damvaeba, S.; Mizunuma, H.; Kwak-Kim, J. Effects of MTHFR C677T Polymorphism on Vitamin D, Homocysteine and Natural Killer Cell Cytotoxicity in Women with Recurrent Pregnancy Losses. Hum. Reprod. 2020, 35, 1276–1287. [Google Scholar] [CrossRef]
- Tan, X.; Yu, Z.; Sao, J.; Chen, L.; Shen, Y.; Ding, J.; Shi, W. Association between in Vitro Fertilization Outcomes and Inherited Thrombophilias: A Meta-Analysis. J. Assist. Reprod. Genet. 2016, 33, 1093–1098. [Google Scholar] [CrossRef]
- Michels, K.A.; Wactawski-Wende, J.; Mills, J.L.; Schliep, K.C.; Gaskins, A.J.; Yeung, E.H.; Kim, K.; Plowden, T.C.; Sjaarda, L.A.; Chaljub, E.N.; et al. Folate, Homocysteine and the Ovarian Cycle among Healthy Regularly Menstruating Women. Hum. Reprod. 2017, 32, 1743–1750. [Google Scholar] [CrossRef]
- Eussen, S.J.P.M.; Nilsen, R.M.; Midttun, Ø.; Hustad, S.; IJssennagger, N.; Meyer, K.; Fredriksen, Å.; Ulvik, A.; Ueland, P.M.; Brennan, P.; et al. North-South Gradients in Plasma Concentrations of B-Vitamins and Other Components of One-Carbon Metabolism in Western Europe: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2013, 110, 363–374. [Google Scholar] [CrossRef]
- Christensen, K.E.; Wu, Q.; Wang, X.; Deng, L.; Caudill, M.A.; Rozen, R. Steatosis in Mice Is Associated with Gender, Folate Intake, and Expression of Genes of One-Carbon Metabolism. J. Nutr. 2010, 140, 1736–1741. [Google Scholar] [CrossRef]
- Hao, H.; d’Alincourt-Salazar, M.; Kelley, K.M.M.; Shatnawi, A.; Mukherjee, S.; Shah, Y.M.; Ratnam, M. Estrogen-Induced and TAFII30-Mediated Gene Repression by Direct Recruitment of the Estrogen Receptor and Co-Repressors to the Core Promoter and Its Reversal by Tamoxifen. Oncogene 2007, 26, 7872–7884. [Google Scholar] [CrossRef]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary Requirements and Role in Brain Development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex Differences in Hepatic One-Carbon Metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef]
- van den Beld, A.W.; Kaufman, J.-M.; Zillikens, M.C.; Lamberts, S.W.; Egan, J.M.; van der Lely, A.J. The Physiology of Endocrine Systems with Ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Silva, A.B.P.; Carreiró, F.; Ramos, F.; Sanches-Silva, A. The Role of Endocrine Disruptors in Female Infertility. Mol. Biol. Rep. 2023, 50, 7069–7088. [Google Scholar] [CrossRef]
- Anawalt, B.D.; Matsumoto, A.M. Aging and Androgens: Physiology and Clinical Implications. Rev. Endocr. Metab. Disord. 2022, 23, 1123–1137. [Google Scholar] [CrossRef]
- Islam, H.; Masud, J.; Islam, Y.N.; Haque, F.K.M. An Update on Polycystic Ovary Syndrome: A Review of the Current State of Knowledge in Diagnosis, Genetic Etiology, and Emerging Treatment Options. Womens Health 2022, 18, 17455057221117966. [Google Scholar] [CrossRef]
- Bøtkjær, J.A.; Kristensen, S.G.; Olesen, H.Ø.; Larsson, P.; Mannaerts, B.; Andersen, C.Y. Dose-Dependent Stimulation of Human Follicular Steroidogenesis by a Novel rhCG during Ovarian Stimulation with Fixed rFSH Dosing. Front. Endocrinol. 2022, 13, 1004596. [Google Scholar] [CrossRef]
- Quaas, A.M. Triggering Change in Stimulation Protocols: A Matter of Oocyte Maturation and Beyond. J. Assist. Reprod. Genet. 2021, 38, 1285–1287. [Google Scholar] [CrossRef]
- Boxmeer, J.C.; Steegers-Theunissen, R.P.M.; Lindemans, J.; Wildhagen, M.F.; Martini, E.; Steegers, E.A.P.; Macklon, N.S. Homocysteine Metabolism in the Pre-Ovulatory Follicle during Ovarian Stimulation. Hum. Reprod. 2008, 23, 2570–2576. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Z.; Lin, P.; Jiang, Z. Association between Serum Homocysteine Level and Unexplained Infertility in in Vitro Fertilization/Intracytoplasmic Sperm Injection (IVF/ICSI): A Retrospective, Hospital-Based, Case-Control Study. J. Clin. Lab. Anal. 2020, 34, e23167. [Google Scholar] [CrossRef]
- Kanakkaparambil, R.; Singh, R.; Li, D.; Webb, R.; Sinclair, K.D. B-Vitamin and Homocysteine Status Determines Ovarian Response to Gonadotropin Treatment in Sheep. Biol. Reprod. 2009, 80, 743–752. [Google Scholar] [CrossRef]
- Laanpere, M.; Altmäe, S.; Stavreus-Evers, A.; Nilsson, T.K.; Yngve, A.; Salumets, A. Folate-Mediated One-Carbon Metabolism and Its Effect on Female Fertility and Pregnancy Viability. Nutr. Rev. 2010, 68, 99–113. [Google Scholar] [CrossRef]
- Bokor, S.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Epigenetic Effect of Maternal Methyl-Group Donor Intake on Offspring’s Health and Disease. Life 2022, 12, 609. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Maternal Methyl Donor Supplementation: A Potential Therapy for Metabolic Disorder in Offspring. J. Nutr. Biochem. 2024, 124, 109533. [Google Scholar] [CrossRef]
- Kwong, W.Y.; Adamiak, S.J.; Gwynn, A.; Singh, R.; Sinclair, K.D. Endogenous Folates and Single-Carbon Metabolism in the Ovarian Follicle, Oocyte and Pre-Implantation Embryo. Reproduction 2010, 139, 705–715. [Google Scholar] [CrossRef]
- Mann, M.R.W.; Watson, A.J. Endogenous Folate Accumulation in Oocytes and Preimplantation Embryos and Its Epigenetic Implications. Biol. Reprod. 2013, 89, 62. [Google Scholar] [CrossRef]
- Golestanfar, A.; Niasari-Naslaji, A.; Jafarpour, F.; Rouhollahi, S.; Rezaei, N.; Menezo, Y.; Dattilo, M.; Nasr-Esfahani, M.H. Metabolic Enhancement of the One Carbon Metabolism (OCM) in Bovine Oocytes IVM Increases the Blastocyst Rate: Evidences for a OCM Checkpoint. Sci. Rep. 2022, 12, 20629. [Google Scholar] [CrossRef]
- Huo, Y.; Yan, Z.Q.; Yuan, P.; Qin, M.; Kuo, Y.; Li, R.; Yan, L.Y.; Feng, H.L.; Qiao, J. Single-Cell DNA Methylation Sequencing Reveals Epigenetic Alterations in Mouse Oocytes Superovulated with Different Dosages of Gonadotropins. Clin. Epigenetics 2020, 12, 75. [Google Scholar] [CrossRef]
- Tang, S.-B.; Yang, L.-L.; Zhang, T.-T.; Wang, Q.; Yin, S.; Luo, S.-M.; Shen, W.; Ge, Z.-J.; Sun, Q.-Y. Multiple Superovulations Alter Histone Modifications in Mouse Early Embryos. Reproduction 2019, 157, 511–523. [Google Scholar] [CrossRef]
- Uysal, F.; Ozturk, S.; Akkoyunlu, G. Superovulation Alters DNA Methyltransferase Protein Expression in Mouse Oocytes and Early Embryos. J. Assist. Reprod. Genet. 2018, 35, 503–513. [Google Scholar] [CrossRef]
- Sato, A.; Otsu, E.; Negishi, H.; Utsunomiya, T.; Arima, T. Aberrant DNA Methylation of Imprinted Loci in Superovulated Oocytes. Hum. Reprod. 2007, 22, 26–35. [Google Scholar] [CrossRef]
- Cannarella, R.; Crafa, A.; Mongioì, L.M.; Leggio, L.; Iraci, N.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. DNA Methylation in Offspring Conceived after Assisted Reproductive Techniques: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5056. [Google Scholar] [CrossRef]
- Gardner, D.K.; Sakkas, D. Making and Selecting the Best Embryo in the Laboratory. Fertil. Steril. 2022, 120, 457–466. [Google Scholar] [CrossRef]
- Bick, L.; Nielsen, A.S.; Knudsen, U.B. Embryo Culture Media Influence on Live Birth Rate and Birthweight after IVF/ICSI: A Systematic Review Comparing Vitrolife G5 Media to Other Common Culture Media. JBRA Assist. Reprod. 2021, 25, 480–492. [Google Scholar] [CrossRef]
- Ermisch, A.F.; Herrick, J.R.; Pasquariello, R.; Dyer, M.C.; Lyons, S.M.; Broeckling, C.D.; Rajput, S.K.; Schoolcraft, W.B.; Krisher, R.L. A Novel Culture Medium with Reduced Nutrient Concentrations Supports the Development and Viability of Mouse Embryos. Sci. Rep. 2020, 10, 9263. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Evans, J.; Nguyen, H.P.T.; Edgell, T.A. The Microenvironment of Human Implantation: Determinant of Reproductive Success. Am. J. Reprod. Immunol. 2016, 75, 218–225. [Google Scholar] [CrossRef]
- Coy, P.; Romar, R.; Romero-Aguirregomezcorta, J. The Embryo Culture Media in the Era of Epigenetics: Is It Time to Go Back to Nature? Anim Reprod 2022, 19, e20210132. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large Offspring Syndrome in Cattle and Sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Nava-Trujillo, H.; Rivera, R.M. Review: Large Offspring Syndrome in Ruminants: Current Status and Prediction during Pregnancy. Animal 2023, 17 (Suppl. S1), 100740. [Google Scholar] [CrossRef]
- Chen, Z.; Robbins, K.M.; Wells, K.D.; Rivera, R.M. Large Offspring Syndrome. Epigenetics 2013, 8, 591–601. [Google Scholar] [CrossRef]
- Young, L.E.; Fernandes, K.; McEvoy, T.G.; Butterwith, S.C.; Gutierrez, C.G.; Carolan, C.; Broadbent, P.J.; Robinson, J.J.; Wilmut, I.; Sinclair, K.D. Epigenetic Change in IGF2R Is Associated with Fetal Overgrowth after Sheep Embryo Culture. Nat. Genet. 2001, 27, 153–154. [Google Scholar] [CrossRef]
- Mussa, A.; Molinatto, C.; Cerrato, F.; Palumbo, O.; Carella, M.; Baldassarre, G.; Carli, D.; Peris, C.; Riccio, A.; Ferrero, G.B. Assisted Reproductive Techniques and Risk of Beckwith-Wiedemann Syndrome. Pediatrics 2017, 140, e20164311. [Google Scholar] [CrossRef]
- Gazzin, A.; Carli, D.; Sirchia, F.; Molinatto, C.; Cardaropoli, S.; Palumbo, G.; Zampino, G.; Ferrero, G.B.; Mussa, A. Phenotype Evolution and Health Issues of Adults with Beckwith-Wiedemann Syndrome. Am. J. Med. Genet. A 2019, 179, 1691–1702. [Google Scholar] [CrossRef]
- Eßinger, C.; Karch, S.; Moog, U.; Fekete, G.; Lengyel, A.; Pinti, E.; Eggermann, T.; Begemann, M. Frequency of KCNQ1 Variants Causing Loss of Methylation of Imprinting Centre 2 in Beckwith-Wiedemann Syndrome. Clin. Epigenetics 2020, 12, 63. [Google Scholar] [CrossRef]
- Kleijkers, S.H.M.; Eijssen, L.M.T.; Coonen, E.; Derhaag, J.G.; Mantikou, E.; Jonker, M.J.; Mastenbroek, S.; Repping, S.; Evers, J.L.H.; Dumoulin, J.C.M.; et al. Differences in Gene Expression Profiles between Human Preimplantation Embryos Cultured in Two Different IVF Culture Media. Hum. Reprod. 2015, 30, 2303–2311. [Google Scholar] [CrossRef]
- Sinclair, K.D.; McEvoy, T.G.; Maxfield, E.K.; Maltin, C.A.; Young, L.E.; Wilmut, I.; Broadbent, P.J.; Robinson, J.J. Aberrant Fetal Growth and Development after in Vitro Culture of Sheep Zygotes. J. Reprod. Fertil. 1999, 116, 177–186. [Google Scholar] [CrossRef]
- Rooke, J.A.; McEvoy, T.G.; Ashworth, C.J.; Robinson, J.J.; Wilmut, I.; Young, L.E.; Sinclair, K.D. Ovine Fetal Development Is More Sensitive to Perturbation by the Presence of Serum in Embryo Culture before Rather than after Compaction. Theriogenology 2007, 67, 639–647. [Google Scholar] [CrossRef]
- Shojaei Saadi, H.A.; Gagné, D.; Fournier, É.; Baldoceda Baldeon, L.M.; Sirard, M.-A.; Robert, C. Responses of Bovine Early Embryos to S-Adenosyl Methionine Supplementation in Culture. Epigenomics 2016, 8, 1039–1060. [Google Scholar] [CrossRef]
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted Reproductive Technologies Are Associated with Limited Epigenetic Variation at Birth That Largely Resolves by Adulthood. Nat. Commun. 2019, 10, 3922. [Google Scholar] [CrossRef]
- Koeck, R.M.; Busato, F.; Tost, J.; Consten, D.; van Echten-Arends, J.; Mastenbroek, S.; Wurth, Y.; Remy, S.; Langie, S.; Nawrot, T.S.; et al. Methylome-Wide Analysis of IVF Neonates That Underwent Embryo Culture in Different Media Revealed No Significant Differences. NPJ Genom. Med. 2022, 7, 39. [Google Scholar] [CrossRef]
- Komiya, A.; Kato, M.; Shibata, H.; Imamura, Y.; Sazuka, T.; Sakamoto, S.; Uchida, N.; Takayanagi, Y.; Nako, Y.; Tajima, M.; et al. Results of Lifestyle Modification Promotion and Reproductive/General Health Check for Male Partners of Couples Seeking Conception. Heliyon 2023, 9, e15203. [Google Scholar] [CrossRef]
- Dadgar, Z.; Shariatzadeh, S.M.A.; Mehranjani, M.S.; Kheirolahi, A. The Therapeutic Effect of Co-Administration of Pentoxifylline and Zinc in Men with Idiopathic Infertility. Ir. J. Med. Sci. 2023, 192, 431–439. [Google Scholar] [CrossRef]
- Chabchoub, I.; Nouioui, M.A.; Araoud, M.; Mabrouk, M.; Amira, D.; Ben Aribia, M.H.; Mahmoud, K.; Zhioua, F.; Merdassi, G.; Hedhili, A. Effects of Lead, Cadmium, Copper and Zinc Levels on the Male Reproductive Function. Andrologia 2021, 53, e14181. [Google Scholar] [CrossRef]
- Wang, L.; Liang, R.; Zhang, G.; Ren, M.; Long, M.; Na, J.; Li, Z.; Wang, B.; Zhuang, L.; Lu, Q. Serum Zinc Concentration and Risk of Adverse Outcomes to in Vitro Fertilization and Embryo Transfer: A Prospective Cohort Study in Northern China. Sci. Total Environ. 2021, 792, 148405. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Clemons, T.; Peterson, C.M.; Johnstone, E.; Hammoud, A.O.; Lamb, D.; Carrell, D.T.; Perkins, N.J.; Sjaarda, L.A.; Van Voorhis, B.J.; et al. A Randomized Trial to Evaluate the Effects of Folic Acid and Zinc Supplementation on Male Fertility and Livebirth: Design and Baseline Characteristics. Am. J. Epidemiol. 2020, 189, 8–26. [Google Scholar] [CrossRef]
- Tulić, L.; Vidaković, S.; Tulić, I.; Ćurčić, M.; Bulat, Z. Toxic Metal and Trace Element Concentrations in Blood and Outcome of In Vitro Fertilization in Women. Biol. Trace Elem. Res. 2019, 188, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Wdowiak, E.; Bojar, I. Evaluation of Trace Metals in Follicular Fluid in ICSI-Treated Patients. Ann. Agric. Environ. Med. 2017, 25, 213–218. [Google Scholar] [CrossRef]
- Berkovitz, A.; Allouche-Fitoussi, D.; Izhakov, D.; Breitbart, H. Cryopreservation of Human Sperm in the Presence of Zn2+ Increases the Motility Rate. J. Obstet. Gynecol. Investig. 2018, 1, 6–12. [Google Scholar] [CrossRef]
- Ingle, M.E.; Bloom, M.S.; Parsons, P.J.; Steuerwald, A.J.; Kruger, P.; Fujimoto, V.Y. Associations between IVF Outcomes and Essential Trace Elements Measured in Follicular Fluid and Urine: A Pilot Study. J. Assist. Reprod. Genet. 2017, 34, 253–261. [Google Scholar] [CrossRef]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing Zinc Oxide Nanoparticles to Cryopreservation Medium Minimizes the Freeze-Thaw-Induced Damage to Spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Giacone, F.; Condorelli, R.A.; Mongioì, L.M.; Bullara, V.; La Vignera, S.; Calogero, A.E. In Vitro Effects of Zinc, D-Aspartic Acid, and Coenzyme-Q10 on Sperm Function. Endocrine 2017, 56, 408–415. [Google Scholar] [CrossRef]
- Nematollahi-Mahani, S.N.; Azizollahi, G.H.; Baneshi, M.R.; Safari, Z.; Azizollahi, S. Effect of Folic Acid and Zinc Sulphate on Endocrine Parameters and Seminal Antioxidant Level after Varicocelectomy. Andrologia 2014, 46, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of Oxidative Stress in Follicular Fluid of Women with Endometriosis and Tubal Infertility Undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef]
- Kotdawala, A.P.; Kumar, S.; Salian, S.R.; Thankachan, P.; Govindraj, K.; Kumar, P.; Kalthur, G.; Adiga, S.K. Addition of Zinc to Human Ejaculate Prior to Cryopreservation Prevents Freeze-Thaw-Induced DNA Damage and Preserves Sperm Function. J. Assist. Reprod. Genet. 2012, 29, 1447–1453. [Google Scholar] [CrossRef]
- Atig, F.; Raffa, M.; Ali, H.B.; Abdelhamid, K.; Saad, A.; Ajina, M. Altered Antioxidant Status and Increased Lipid Per-Oxidation in Seminal Plasma of Tunisian Infertile Men. Int. J. Biol. Sci. 2012, 8, 139–149. [Google Scholar] [CrossRef]
- Dickerson, E.H.; Sathyapalan, T.; Knight, R.; Maguiness, S.M.; Killick, S.R.; Robinson, J.; Atkin, S.L. Endocrine Disruptor & Nutritional Effects of Heavy Metals in Ovarian Hyperstimulation. J. Assist. Reprod. Genet. 2011, 28, 1223–1228. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc Levels in Seminal Plasma Are Associated with Sperm Quality in Fertile and Infertile Men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Azemi, M.K.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Mathew, T.C. Indications of the Mechanisms Involved in Improved Sperm Parameters by Zinc Therapy. Med. Princ. Pract. 2008, 17, 108–116. [Google Scholar] [CrossRef]
- Ebisch, I.M.W.; Pierik, F.H.; DE Jong, F.H.; Thomas, C.M.G.; Steegers-Theunissen, R.P.M. Does Folic Acid and Zinc Sulphate Intervention Affect Endocrine Parameters and Sperm Characteristics in Men? Int. J. Androl. 2006, 29, 339–345. [Google Scholar] [CrossRef]
- Benoff, S.; Cooper, G.W.; Paine, T.; Hurley, I.R.; Napolitano, B.; Jacob, A.; Scholl, G.M.; Hershlag, A. Numerical Dose-Compensated in Vitro Fertilization Inseminations Yield High Fertilization and Pregnancy Rates. Fertil. Steril. 1999, 71, 1019–1028. [Google Scholar] [CrossRef]
- Tikkiwal, M.; Ajmera, R.L.; Mathur, N.K. Effect of Zinc Administration on Seminal Zinc and Fertility of Oligospermic Males. Indian. J. Physiol. Pharmacol. 1987, 31, 30–34. [Google Scholar]
- Tian, X.; Diaz, F.J. Acute Dietary Zinc Deficiency before Conception Compromises Oocyte Epigenetic Programming and Disrupts Embryonic Development. Dev. Biol. 2013, 376, 51–61. [Google Scholar] [CrossRef]
- Geravandi, S.; Karami, A.; Azadbakht, M.; Kalehoei, E.; Nowrouzi, F.; Bakhatiari, M. Follicular Fluid Supplemented with Copper and Zinc Increase the Maturation Rate of Mouse Vitrified-Warmed Oocytes. Cryo Lett. 2021, 42, 326–331. [Google Scholar]
- Janati, S.; Behmanesh, M.A.; Najafzadehvarzi, H.; Akhundzade, Z.; Poormoosavi, S.M. Follicular Fluid Zinc Level and Oocyte Maturity and Embryo Quality in Women with Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2021, 15, 197–201. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Yang, S.-J.; Ahmad, M.J.; Ding, Z.-M.; Duan, Z.-Q.; Chen, Y.-W.; Liu, M.; Liang, A.-X.; Hua, G.-H.; Huo, L.-J. Zinc Pyrithione Exposure Compromises Oocyte Maturation through Involving in Spindle Assembly and Zinc Accumulation. Ecotoxicol. Environ. Saf. 2022, 234, 113393. [Google Scholar] [CrossRef]
- Uh, K.; Hay, A.; Chen, P.; Reese, E.; Lee, K. Design of Novel Oocyte Activation Methods: The Role of Zinc. Biol. Reprod. 2022, 106, 264–273. [Google Scholar] [CrossRef]
- Galarza, E.M.; Lizarraga, R.M.; Anchordoquy, J.P.; Farnetano, N.A.; Furnus, C.C.; Fazzio, L.E.; Anchordoquy, J.M. Zinc Supplementation within the Reference Ranges for Zinc Status in Cattle Improves Sperm Quality without Modifying in Vitro Fertilization Performance. Anim. Reprod. Sci. 2020, 221, 106595. [Google Scholar] [CrossRef]
- Anchordoquy, J.P.; Anchordoquy, J.M.; Lizarraga, R.M.; Nikoloff, N.; Pascua, A.M.; Furnus, C.C. The Importance of Trace Minerals Copper, Manganese, Selenium and Zinc in Bovine Sperm-Zona Pellucida Binding. Zygote 2019, 27, 89–96. [Google Scholar] [CrossRef]
- Zhang, N.; Duncan, F.E.; Que, E.L.; O’Halloran, T.V.; Woodruff, T.K. The Fertilization-Induced Zinc Spark Is a Novel Biomarker of Mouse Embryo Quality and Early Development. Sci. Rep. 2016, 6, 22772. [Google Scholar] [CrossRef]
- Jenkins, T.; Aston, K.; Carrell, D.; DeVilbiss, E.; Sjaarda, L.; Perkins, N.; Mills, J.L.; Chen, Z.; Sparks, A.; Clemons, T.; et al. The Impact of Zinc and Folic Acid Supplementation on Sperm DNA Methylation: Results from the Folic Acid and Zinc Supplementation Randomized Clinical Trial (FAZST). Fertil. Steril. 2022, 117, 75–85. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Halo, M.; Bułka, K.; Antos, P.A.; Greń, A.; Slanina, T.; Ondruška, Ľ.; Tokárová, K.; Massányi, M.; Formicki, G.; Halo, M.; et al. The Effect of ZnO Nanoparticles on Rabbit Spermatozoa Motility and Viability Parameters in Vitro. Saudi J. Biol. Sci. 2021, 28, 7450–7454. [Google Scholar] [CrossRef]
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Foresta, C. Dietary Supplements for Male Infertility: A Critical Evaluation of Their Composition. Nutrients 2020, 12, E1472. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling Pathways Involved in Human Sperm Hyperactivated Motility Stimulated by Zn2. Mol. Reprod. Dev. 2018, 85, 543–556. [Google Scholar] [CrossRef]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-Stimulation of Sperm Capacitation and of the Acrosome Reaction Is Mediated by EGFR Activation. Dev. Biol. 2014, 396, 246–255. [Google Scholar] [CrossRef]
- Kherraf, Z.-E.; Cazin, C.; Lestrade, F.; Muronova, J.; Coutton, C.; Arnoult, C.; Thierry-Mieg, N.; Ray, P.F. From Azoospermia to Macrozoospermia, a Phenotypic Continuum Due to Mutations in the ZMYND15 Gene. Asian J. Androl. 2022, 24, 243–247. [Google Scholar] [CrossRef]
- Foresta, C.; Garolla, A.; Cosci, I.; Menegazzo, M.; Ferigo, M.; Gandin, V.; De Toni, L. Role of Zinc Trafficking in Male Fertility: From Germ to Sperm. Hum. Reprod. 2014, 29, 1134–1145. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Fleva, A.; Sioga, A.; Georgiou, I.; Nikolopoulos, T.-T.; Markopoulou, M.; Petrogiannis, N.; Anifandis, G.; Patrikiou, A.; Kolibianakis, E.; et al. Effects of Different Drug Therapies and COVID-19 mRNA Vaccination on Semen Quality in a Man with Ankylosing Spondylitis: A Case Report. Medicina 2022, 58, 173. [Google Scholar] [CrossRef]
- Wu, J.; Wu, S.; Xie, Y.; Wang, Z.; Wu, R.; Cai, J.; Luo, X.; Huang, S.; You, L. Zinc Protects Sperm from Being Damaged by Reactive Oxygen Species in Assisted Reproduction Techniques. Reprod. Biomed. Online 2015, 30, 334–339. [Google Scholar] [CrossRef]
- Khodaei-Motlagh, M.; Masoudi, R.; Karimi-Sabet, M.J.; Hatefi, A. Supplementation of Sperm Cooling Medium with Zinc and Zinc Oxide Nanoparticles Preserves Rooster Sperm Quality and Fertility Potential. Theriogenology 2022, 183, 36–40. [Google Scholar] [CrossRef]
- Zakošek Pipan, M.; Zrimšek, P.; Jakovac Strajn, B.; Pavšič Vrtač, K.; Knific, T.; Mrkun, J. Macro- and Microelements in Serum and Seminal Plasma as Biomarkers for Bull Sperm Cryotolerance. Acta Vet. Scand. 2021, 63, 25. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc Deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Balashova, O.A.; Visina, O.; Borodinsky, L.N. Folate Action in Nervous System Development and Disease. Dev. Neurobiol. 2018, 78, 391–402. [Google Scholar] [CrossRef]
- Paffoni, A.; Reschini, M.; Noli, S.A.; Viganò, P.; Parazzini, F.; Somigliana, E. Folate Levels and Pregnancy Rate in Women Undergoing Assisted Reproductive Techniques: A Systematic Review and Meta-Analysis. Reprod. Sci. 2022, 29, 341–356. [Google Scholar] [CrossRef]
- Polzikov, M.; Blinov, D.; Barakhoeva, Z.; Vovk, L.; Fetisova, Y.; Ovchinnikova, M.; Tischenko, M.; Zorina, I.; Yurasov, V.; Ushakova, T.; et al. Association of the Serum Folate and Total Calcium and Magnesium Levels Before Ovarian Stimulation with Outcomes of Fresh In Vitro Fertilization Cycles in Normogonadotropic Women. Front. Endocrinol. 2022, 13, 732731. [Google Scholar] [CrossRef]
- De Cosmi, V.; Cipriani, S.; Esposito, G.; Fedele, F.; La Vecchia, I.; Trojano, G.; Parazzini, F.; Somigliana, E.; Agostoni, C. Vitamin and Carotenoid Intake and Outcomes of In Vitro Fertilization in Women Referring to an Italian Fertility Service: A Cross-Sectional Analysis of a Prospective Cohort Study. Antioxidants 2023, 12, 286. [Google Scholar] [CrossRef]
- Tabatabaie, M.; Amiri, S.; Golestan Jahromi, M.; Sene, A.A.; Zandieh, Z.; Mehdizadeh, M.; Amjadi, F. The Effect of Myo-Inositol Supplement on Molecular Regulation of Folliculogenesis, Steroidogenesis, and Assisted Reproductive Technique Outcomes in Patients with Polycystic Ovarian Syndrome. Mol. Biol. Rep. 2022, 49, 875–884. [Google Scholar] [CrossRef]
- Mathieu d’Argent, E.; Ravel, C.; Rousseau, A.; Morcel, K.; Massin, N.; Sussfeld, J.; Simon, T.; Antoine, J.-M.; Mandelbaume, J.; Daraï, E.; et al. High-Dose Supplementation of Folic Acid in Infertile Men Improves IVF-ICSI Outcomes: A Randomized Controlled Trial (FOLFIV Trial). J. Clin. Med. 2021, 10, 1876. [Google Scholar] [CrossRef]
- Mohammadi, S.; Eini, F.; Bazarganipour, F.; Taghavi, S.A.; Kutenaee, M.A. The Effect of Myo-Inositol on Fertility Rates in Poor Ovarian Responder in Women Undergoing Assisted Reproductive Technique: A Randomized Clinical Trial. Reprod. Biol. Endocrinol. 2021, 19, 61. [Google Scholar] [CrossRef]
- So, S.; Yamaguchi, W.; Murabayashi, N.; Miyano, N.; Tawara, F.; Kanayama, N. Beneficial Effect of L-Arginine in Women Using Assisted Reproductive Technologies: A Small-Scale Randomized Controlled Trial. Nutr. Res. 2020, 82, 67–73. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Saharkhiz, N.; Azizi, E.; Hashemi, T.; Ghodssi-Ghassemabadi, R. Effect of Myo-Inositol Supplementation on ICSI Outcomes among Poor Ovarian Responder Patients: A Randomized Controlled Trial. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101698. [Google Scholar] [CrossRef]
- Regidor, P.-A.; Schindler, A.E.; Lesoine, B.; Druckman, R. Management of Women with PCOS Using Myo-Inositol and Folic Acid. New Clinical Data and Review of the Literature. Horm. Mol. Biol. Clin. Investig. 2018, 34, 20170067. [Google Scholar] [CrossRef]
- Nouri, K.; Walch, K.; Weghofer, A.; Imhof, M.; Egarter, C.; Ott, J. The Impact of a Standardized Oral Multinutrient Supplementation on Embryo Quality in in Vitro Fertilization/Intracytoplasmic Sperm Injection: A Prospective Randomized Trial. Gynecol. Obstet. Investig. 2017, 82, 8–14. [Google Scholar] [CrossRef]
- Murto, T.; Yngve, A.; Skoog Svanberg, A.; Altmäe, S.; Salumets, A.; Wånggren, K.; Stavreus-Evers, A. Compliance to the Recommended Use of Folic Acid Supplements for Women in Sweden Is Higher among Those under Treatment for Infertility than among Fertile Controls and Is Also Related to Socioeconomic Status and Lifestyle. Food Nutr. Res. 2017, 61, 1334483. [Google Scholar] [CrossRef]
- D’Elia, P.Q.; dos Santos, A.A.; Bianco, B.; Barbosa, C.P.; Christofolini, D.M.; Aoki, T. MTHFR Polymorphisms C677T and A1298C and Associations with IVF Outcomes in Brazilian Women. Reprod. Biomed. Online 2014, 28, 733–738. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Li, Q.; Chen, L.-X.; Tian, T.; Kang, J.; Hao, Y.-X.; Zhou, J.-S.; Wang, Y.-Y.; Yan, L.-Y.; Li, R.; et al. Association between Maternal MTHFR C677T/A1298C Combination Polymorphisms and IVF/ICSI Outcomes: A Retrospective Cohort Study. Human. Reprod. Open 2023, 2023, hoac055. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, V.; Ansari, S.; Kumar, A.; Thakur, A.; Malik, H.; Kumar, S.; Malakar, D. Folate Supplementation during Oocyte Maturation Positively Impacts the Folate-Methionine Metabolism in Pre-Implantation Embryos. Theriogenology 2022, 182, 63–70. [Google Scholar] [CrossRef]
- Gennari Verruma, C.; Credendio Eiras, M.; Fernandes, A.; Vila, R.A.; Libardi Miranda Furtado, C.; Silveira Ramos, E.; Barbosa Lôbo, R. Folic Acid Supplementation during Oocytes Maturation Influences in Vitro Production and Gene Expression of Bovine Embryos. Zygote 2021, 29, 342–349. [Google Scholar] [CrossRef]
- Jiang, Q.; Lu, D.; Wang, F.; Zhang, Y.; Cao, L.; Gui, Y.; Sun, S. Folic Acid Supplement Rescues Ethanol-Induced Developmental Defects in the Zebrafish Embryos. Acta Biochim. Biophys. Sin. 2020, 52, 536–545. [Google Scholar] [CrossRef]
- Dong, J.; Yin, L.-L.; Deng, X.-D.; Ji, C.-Y.; Pan, Q.; Yang, Z.; Peng, T.; Wu, J.-N.; Early Pregnancy Ultrasound Screening, Maternal Exposures and Congenital Malformation Risk collaborators. Initiation and Duration of Folic Acid Supplementation in Preventing Congenital Malformations. BMC Med. 2023, 21, 292. [Google Scholar] [CrossRef]
- Geraghty, A.A.; Lindsay, K.L.; Alberdi, G.; McAuliffe, F.M.; Gibney, E.R. Nutrition During Pregnancy Impacts Offspring’s Epigenetic Status-Evidence from Human and Animal Studies. Nutr. Metab. Insights 2015, 8, 41–47. [Google Scholar] [CrossRef]
- Boughanem, H.; Hernandez-Alonso, P.; Tinahones, A.; Babio, N.; Salas-Salvadó, J.; Tinahones, F.J.; Macias-Gonzalez, M. Association between Serum Vitamin B12 and Global DNA Methylation in Colorectal Cancer Patients. Nutrients 2020, 12, E3567. [Google Scholar] [CrossRef]
- Mahajan, A.; Sapehia, D.; Thakur, S.; Mohanraj, P.S.; Bagga, R.; Kaur, J. Effect of Imbalance in Folate and Vitamin B12 in Maternal/Parental Diet on Global Methylation and Regulatory miRNAs. Sci. Rep. 2019, 9, 17602. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Chiu, Y.-H.; Williams, P.L.; Ford, J.B.; Toth, T.L.; Hauser, R.; Chavarro, J.E.; EARTH Study Team. Association between Serum Folate and Vitamin B-12 and Outcomes of Assisted Reproductive Technologies. Am. J. Clin. Nutr. 2015, 102, 943–950. [Google Scholar] [CrossRef]
- El-Nemr, A.; Sabatini, L.; Wilson, C.; Lower, A.M.; Al-Shawaf, T.; Grudzinskas, J.G. Vitamin B12 Deficiency and IVF. J. Obstet. Gynaecol. 1998, 18, 192–193. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Nassan, F.L.; Chiu, Y.-H.; Arvizu, M.; Williams, P.L.; Keller, M.G.; Souter, I.; Hauser, R.; Chavarro, J.E.; EARTH Study Team. Dietary Patterns and Outcomes of Assisted Reproduction. Am. J. Obstet. Gynecol. 2019, 220, 567.e1–567.e18. [Google Scholar] [CrossRef]
- Schaefer, E.; Nock, D. The Impact of Preconceptional Multiple-Micronutrient Supplementation on Female Fertility. Clin. Med. Insights Womens Health 2019, 12, 1179562X19843868. [Google Scholar] [CrossRef]
- Rogenhofer, N.; Mischitz, D.; Mann, C.; Gluderer, J.; von Schönfeldt, V.; Jeschke, U.; Thaler, C.J. Correlation of Vitamin D3 (Calcitriol) Serum Concentrations with Vitamin B12 and Folic Acid in Women Undergoing In Vitro Fertilisation/Intracytoplasmatic Sperm Injection. Gynecol. Obstet. Investig. 2019, 84, 128–135. [Google Scholar] [CrossRef]
- Cirillo, M.; Fucci, R.; Rubini, S.; Coccia, M.E.; Fatini, C. 5-Methyltetrahydrofolate and Vitamin B12 Supplementation Is Associated with Clinical Pregnancy and Live Birth in Women Undergoing Assisted Reproductive Technology. Int. J. Environ. Res. Public. Health 2021, 18, 12280. [Google Scholar] [CrossRef]
- La Vecchia, I.; Paffoni, A.; Castiglioni, M.; Ferrari, S.; Bortolus, R.; Ferraris Fusarini, C.; Bettinardi, N.; Somigliana, E.; Parazzini, F. Folate, Homocysteine and Selected Vitamins and Minerals Status in Infertile Women. Eur. J. Contracept. Reprod. Health Care 2017, 22, 70–75. [Google Scholar] [CrossRef]
- Sundrani, D.; Khot, V.; Joshi, S. Chapter 18—DNA Methylation for Prediction of Adverse Pregnancy Outcomes. In Epigenetic Biomarkers and Diagnostics; García-Giménez, J.L., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 351–376. ISBN 978-0-12-801899-6. [Google Scholar]
- Estrada-Cortés, E.; Ortiz, W.; Rabaglino, M.B.; Block, J.; Rae, O.; Jannaman, E.A.; Xiao, Y.; Hansen, P.J. Choline Acts during Preimplantation Development of the Bovine Embryo to Program Postnatal Growth and Alter Muscle DNA Methylation. FASEB J. 2021, 35, e21926. [Google Scholar] [CrossRef]
- Yurci, A.; Dokuzeylul Gungor, N.; Gurbuz, T. Spectroscopy Analysis of Endometrial Metabolites Is a Powerful Predictor of Success of Embryo Transfer in Women with Implantation Failure: A Preliminary Study. Gynecol. Endocrinol. 2021, 37, 415–421. [Google Scholar] [CrossRef]
- Dokuzeylül Güngör, N.; Güngör, K. Ovarian Stimulation Drugs Alter the Metabolite Content of the Growing Follicle: In Vivo Spectroscopic Evaluation of Follicle Fluid. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 132–138. [Google Scholar] [CrossRef]
- Nagy, R.A.; Homminga, I.; Jia, C.; Liu, F.; Anderson, J.L.C.; Hoek, A.; Tietge, U.J.F. Trimethylamine-N-Oxide Is Present in Human Follicular Fluid and Is a Negative Predictor of Embryo Quality. Hum. Reprod. 2020, 35, 81–88. [Google Scholar] [CrossRef]
- Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. An Investigation into the Relationship between the Metabolic Profile of Follicular Fluid, Oocyte Developmental Potential, and Implantation Outcome. Fertil. Steril. 2012, 97, 1078–1084.e8. [Google Scholar] [CrossRef]
- Reynolds, S.; Calvert, S.J.; Walters, S.J.; Paley, M.N.; Pacey, A.A. NMR Spectroscopy of Live Human Asthenozoospermic and Normozoospermic Sperm Metabolism. Reprod. Fertil. 2022, 3, 77–89. [Google Scholar] [CrossRef]
- Lazaros, L.; Xita, N.; Hatzi, E.; Kaponis, A.; Makrydimas, G.; Takenaka, A.; Sofikitis, N.; Stefos, T.; Zikopoulos, K.; Georgiou, I. Phosphatidylethanolamine N-Methyltransferase and Choline Dehydrogenase Gene Polymorphisms Are Associated with Human Sperm Concentration. Asian J. Androl. 2012, 14, 778–783. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wen, Y.; Zhang, T.; Wang, X.; Jiang, C.; Zheng, R.; Zhou, F.; Chen, D.; Yang, Y.; et al. Whole-Exome Sequencing of a Cohort of Infertile Men Reveals Novel Causative Genes in Teratozoospermia That Are Chiefly Related to Sperm Head Defects. Hum. Reprod. 2021, 37, 152–177. [Google Scholar] [CrossRef]
- Quintans, C.J.; Donaldson, M.J.; Bertolino, M.V.; Pasqualini, R.S. Birth of Two Babies Using Oocytes That Were Cryopreserved in a Choline-Based Freezing Medium. Hum. Reprod. 2002, 17, 3149–3152. [Google Scholar] [CrossRef]
- Li, Z.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewell, L.; Mason, A.; Vance, D.E. The Ratio of Phosphatidylcholine to Phosphatidylethanolamine Influences Membrane Integrity and Steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef]
- Zeisel, S.H. Importance of Methyl Donors during Reproduction. Am. J. Clin. Nutr. 2009, 89, 673S–677S. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.L.; Akkaya-Hocagil, T.; Ryan, L.M.; Dodge, N.C.; Richardson, G.A.; Olson, H.C.; Coles, C.D.; Day, N.L.; Cook, R.J.; Jacobson, S.W. Effects of Prenatal Alcohol Exposure on Cognitive and Behavioral Development: Findings from a Hierarchical Meta-Analysis of Data from Six Prospective Longitudinal U.S. Cohorts. Alcohol. Clin. Exp. Res. 2021, 45, 2040–2058. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Scaruffi, P.; Licata, E.; Maccarini, E.; Massarotti, C.; Bovis, F.; Sozzi, F.; Stigliani, S.; Dal Lago, A.; Casciano, I.; Rago, R.; et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. J. Clin. Med. 2021, 10, 3254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jing, H.; Dou, C.; Zhang, L.; Wu, X.; Wu, Q.; Song, H.; Li, D.; Wu, F.; Liu, Y.; et al. Supplement of Betaine into Embryo Culture Medium Can Rescue Injury Effect of Ethanol on Mouse Embryo Development. Sci. Rep. 2018, 8, 1761. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.Y.; Lee, D.Y.; Park, K.J.; Kim, G.H.; Kim, J.E.; Roh, G.S.; Lim, J.Y.; Koo, S.; Lim, N.K.; et al. Alcohol Consumption before Pregnancy Causes Detrimental Fetal Development and Maternal Metabolic Disorders. Sci. Rep. 2020, 10, 10054. [Google Scholar] [CrossRef] [PubMed]
- Garro, A.J.; McBeth, D.L.; Lima, V.; Lieber, C.S. Ethanol Consumption Inhibits Fetal DNA Methylation in Mice: Implications for the Fetal Alcohol Syndrome. Alcohol. Clin. Exp. Res. 1991, 15, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, G.; Sheehan, M.M.; Doughman, Y.Q.; Gu, S.; Sun, J.; Li, Y.; Strainic, J.P.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Supplementation with the Methyl Donor Betaine Prevents Congenital Defects Induced by Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2017, 41, 1917–1927. [Google Scholar] [CrossRef]
- Berker, B.; Kaya, C.; Aytac, R.; Satiroglu, H. Homocysteine Concentrations in Follicular Fluid Are Associated with Poor Oocyte and Embryo Qualities in Polycystic Ovary Syndrome Patients Undergoing Assisted Reproduction. Hum. Reprod. 2009, 24, 2293–2302. [Google Scholar] [CrossRef]
- Ocal, P.; Ersoylu, B.; Cepni, I.; Guralp, O.; Atakul, N.; Irez, T.; Idil, M. The Association between Homocysteine in the Follicular Fluid with Embryo Quality and Pregnancy Rate in Assisted Reproductive Techniques. J. Assist. Reprod. Genet. 2012, 29, 299–304. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, E1421. [Google Scholar] [CrossRef]
- Nelen, W.L.; Bulten, J.; Steegers, E.A.; Blom, H.J.; Hanselaar, A.G.; Eskes, T.K. Maternal Homocysteine and Chorionic Vascularization in Recurrent Early Pregnancy Loss. Hum. Reprod. 2000, 15, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Maggiano, N.; Caliandro, D.; Riccardi, P.; Evangelista, A.; Carducci, B.; Caruso, A. Homocysteine Induces Trophoblast Cell Death with Apoptotic Features. Biol. Reprod. 2003, 69, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Razi, Y.; Eftekhar, M.; Fesahat, F.; Dehghani Firouzabadi, R.; Razi, N.; Sabour, M.; Razi, M.H. Concentrations of Homocysteine in Follicular Fluid and Embryo Quality and Oocyte Maturity in Infertile Women: A Prospective Cohort. J. Obstet. Gynaecol. 2021, 41, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Boyama, B.A.; Cepni, I.; Imamoglu, M.; Oncul, M.; Tuten, A.; Yuksel, M.A.; Kervancioglu, M.E.; Kaleli, S.; Ocal, P. Homocysteine in Embryo Culture Media as a Predictor of Pregnancy Outcome in Assisted Reproductive Technology. Gynecol. Endocrinol. 2016, 32, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Manzur, N.F.; Gluska, H.; Feferkorn, I.; Skvirsky, S.; Ben-Shlomo, I.; Wiener-Megnazi, Z. Homocysteine Serum Levels Correlate with the Number of Failed IVF Cycles Even When within Normal Range. Arch. Gynecol. Obstet. 2023, 307, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Han, A.; Jiang, S.; Cao, D.; Jiang, Y.; Sun, L.; Zou, N.; Tao, S.; Xue, X.; Shao, X.; et al. Homocysteine Level Related to Age Is Associated With Embryo Quality in Women Who Had IVF With Diminished Ovarian Reserve. Front. Reprod. Health 2022, 4, 886277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, H.; Wang, X.; Wei, B.; Wu, Z.; Chen, S.; Wang, B.; Huang, H.; Jin, L. Association of Homocysteine with IVF/ICSI Outcomes Stratified by MTHFR C677T Polymorphisms: A Prospective Cohort Study. Reprod. Biomed. Online 2021, 43, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, D.; Wang, Q.; Ma, Y.; Ping, L.; Wu, T.; Tang, J.; Peng, D.; Zhao, P. Serum Homocysteine and Folate Concentrations in Early Pregnancy and Subsequent Events of Adverse Pregnancy Outcome: The Sichuan Homocysteine Study. BMC Pregnancy Childbirth 2020, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Fiscus, J.; Fraison, É.; Renault, L.; Salle, B.; Panthu, B.; Labrune, E. Metabolic Signature of Follicular Fluid in Infertility-Related Diseases: A Narrative Review. Reprod. Biomed. Online 2023, 48, 103762. [Google Scholar] [CrossRef]
- Victor Oluwaloseyi, A.; Aduragbemi Noah, O.; Lydia Oluwatoyin, A.; Gaffar, Y.; Moses, O.; Oyedayo Phillips, A.; Comfort Onaolapo, M.; Sylvester Olateju, B.; Ademola Ayodele, A.; Mega Obukohwo, O.; et al. Metabolomics of Male Infertility. Clin. Chim. Acta 2024, 556, 117850. [Google Scholar] [CrossRef]
- Meng, H.; Huang, S.; Diao, F.; Gao, C.; Zhang, J.; Kong, L.; Gao, Y.; Jiang, C.; Qin, L.; Chen, Y.; et al. Rapid and Non-Invasive Diagnostic Techniques for Embryonic Developmental Potential: A Metabolomic Analysis Based on Raman Spectroscopy to Identify the Pregnancy Outcomes of IVF-ET. Front. Cell Dev. Biol. 2023, 11, 1164757. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, Y. Metabolomics and Its Applications in Assisted Reproductive Technology. IET Nanobiotechnol. 2023, 17, 399–405. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Damblon, C.; Elena-Herrmann, B.; Panthu, B.; Rautureau, G.J.P. Metabolomic Approaches to Study Chemical Exposure-Related Metabolism Alterations in Mammalian Cell Cultures. Int. J. Mol. Sci. 2020, 21, 6843. [Google Scholar] [CrossRef] [PubMed]
- Brinca, A.T.; Ramalhinho, A.C.; Sousa, Â.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Gallardo, E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Castiglione Morelli, M.A.; Iuliano, A.; Schettini, S.C.A.; Petruzzi, D.; Ferri, A.; Colucci, P.; Viggiani, L.; Cuviello, F.; Ostuni, A. NMR Metabolic Profiling of Follicular Fluid for Investigating the Different Causes of Female Infertility: A Pilot Study. Metabolomics 2019, 15, 19. [Google Scholar] [CrossRef]
- Karaer, A.; Tuncay, G.; Mumcu, A.; Dogan, B. Metabolomics Analysis of Follicular Fluid in Women with Ovarian Endometriosis Undergoing in Vitro Fertilization. Syst. Biol. Reprod. Med. 2019, 65, 39–47. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Z.; Zhang, Y.; Li, J.; Ruan, X.; Wan, Q.; Yin, T.; Zou, Y.; Chen, S.; Zhang, Y. Nontargeted Metabolomics Analysis of Follicular Fluid in Patients with Endometriosis Provides a New Direction for the Study of Oocyte Quality. MedComm 2023, 4, e302. [Google Scholar] [CrossRef]
- Pocate-Cheriet, K.; Santulli, P.; Kateb, F.; Bourdon, M.; Maignien, C.; Batteux, F.; Chouzenoux, S.; Patrat, C.; Wolf, J.P.; Bertho, G.; et al. The Follicular Fluid Metabolome Differs According to the Endometriosis Phenotype. Reprod. Biomed. Online 2020, 41, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to Know Endometriosis-Related Infertility Better: A Review on How Endometriosis Affects Oocyte Quality and Embryo Development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef]
- Hou, E.; Zhao, Y.; Hang, J.; Qiao, J. Metabolomics and Correlation Network Analysis of Follicular Fluid Reveals Associations between L-Tryptophan, l-Tyrosine and Polycystic Ovary Syndrome. Biomed. Chromatogr. 2021, 35, e4993. [Google Scholar] [CrossRef]
- Liu, R.; Bai, S.; Zheng, S.; Zhu, X.; Zhang, Y.; Xu, B.; Zhao, W. Identification of the Metabolomics Signature of Human Follicular Fluid from PCOS Women with Insulin Resistance. Dis. Markers 2022, 2022, 6877541. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Li, S.; Zhang, Y.; Feng, R.; Huang, R.; Chen, M.; Qian, Y. Metabolic Signatures in Human Follicular Fluid Identify Lysophosphatidylcholine as a Predictor of Follicular Development. Commun. Biol. 2022, 5, 763. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tu, M.; Qian, Y.; Ma, J.; Chen, L.; Liu, Y.; Wu, Y.; Chen, K.; Liu, J.; Ying, Y.; et al. Age-Dependent Metabolomic Profile of the Follicular Fluids from Women Undergoing Assisted Reproductive Technology Treatment. Front. Endocrinol. 2022, 13, 818888. [Google Scholar] [CrossRef]
- Dogan, B.; Karaer, A.; Tuncay, G.; Tecellioglu, N.; Mumcu, A. High-Resolution 1H-NMR Spectroscopy Indicates Variations in Metabolomics Profile of Follicular Fluid from Women with Advanced Maternal Age. J. Assist. Reprod. Genet. 2020, 37, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Yu, Y.; Sun, Z.-G.; Song, J.-Y.; Wang, A.-J. Metabolomic Analysis of Human Follicular Fluid: Potential Follicular Fluid Markers of Reproductive Aging. J. Pak. Med. Assoc. 2018, 68, 1769–1781. [Google Scholar] [PubMed]
- Lahimer, M.; Abou Diwan, M.; Montjean, D.; Cabry, R.; Bach, V.; Ajina, M.; Ben Ali, H.; Benkhalifa, M.; Khorsi-Cauet, H. Endocrine Disrupting Chemicals and Male Fertility: From Physiological to Molecular Effects. Front. Public. Health 2023, 11, 1232646. [Google Scholar] [CrossRef]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.-I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public. Health 2021, 18, 1464. [Google Scholar] [CrossRef]
- Peña, F.J.; Ortiz-Rodríguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martín-Cano, F.E. An Integrated Overview on the Regulation of Sperm Metabolism (Glycolysis-Krebs Cycle-Oxidative Phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Karkada, I.R.; Chinni, S.V. Endocrinopathies and Male Infertility. Life 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Eirefelt, S.; Stahlhut, M.; Svitacheva, N.; Carnerup, M.A.; Da Rosa, J.M.C.; Ewald, D.A.; Marstrand, T.T.; Krogh-Madsen, M.; Dünstl, G.; Dack, K.N.; et al. Characterization of a Novel Non-Steroidal Glucocorticoid Receptor Agonist Optimized for Topical Treatment. Sci. Rep. 2022, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Correnti, S.; Preianò, M.; Fregola, A.; Gamboni, F.; Stephenson, D.; Savino, R.; D’Alessandro, A.; Terracciano, R. Seminal Plasma Untargeted Metabolomic and Lipidomic Profiling for the Identification of a Novel Panel of Biomarkers and Therapeutic Targets Related to Male Infertility. Front. Pharmacol. 2023, 14, 1275832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Z.; Chen, M.; Xia, Y.; Martin, F.L.; Hang, W.; Shen, H. Urinary Metabolome Identifies Signatures of Oligozoospermic Infertile Men. Fertil. Steril. 2014, 102, 44–53.e12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, H.; Wang, Y.; Zhang, Z.; Wu, Q. Comprehensive Metabolic Profiles of Seminal Plasma with Different Forms of Male Infertility and Their Correlation with Sperm Parameters. J. Pharm. Biomed. Anal. 2020, 177, 112888. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, A.; Rozen, G.; Gyngell, C.; Savulescu, J. Novel Embryo Selection Strategies—Finding the Right Balance. Front. Reprod. Health 2023, 5, 1287621. [Google Scholar] [CrossRef] [PubMed]
- Pierson, H.E.; Invik, J.; Meriano, J.; Pierson, R.A. A Novel System for Rapid Conversion of Gardner Embryo Grades to Linear Scale Numeric Variables. Reprod. Biomed. Online 2023, 46, 808–818. [Google Scholar] [CrossRef]
- Giménez, C.; Conversa, L.; Murria, L.; Meseguer, M. Time-Lapse Imaging: Morphokinetic Analysis of in Vitro Fertilization Outcomes. Fertil. Steril. 2023, 120, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Austin, C.; Warty, R.R.; Tiktin, C.; Rolnik, D.L.; Momeni, M.; Rezatofighi, H.; Reddy, S.; Smith, V.; Vollenhoven, B.; et al. Embryo Selection through Artificial Intelligence versus Embryologists: A Systematic Review. Hum. Reprod. Open 2023, 2023, hoad031. [Google Scholar] [CrossRef]
- Dabbagh Rezaeiye, R.; Mehrara, A.; Ali Pour, A.M.; Fallahi, J.; Forouhari, S. Impact of Various Parameters as Predictors of The Success Rate of In Vitro Fertilization. Int. J. Fertil. Steril. 2022, 16, 76–84. [Google Scholar] [CrossRef]
- Ferrick, L.; Lee, Y.S.L.; Gardner, D.K. Reducing Time to Pregnancy and Facilitating the Birth of Healthy Children through Functional Analysis of Embryo Physiology †. Biol. Reprod. 2019, 101, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.P.; Philippon, A.; Menezo, Y. In-Vitro Uptake of Glucose by Bovine Blastocysts. J. Reprod. Fertil. 1980, 58, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Uyar, A.; Seli, E. Metabolomic Assessment of Embryo Viability. Semin. Reprod. Med. 2014, 32, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Gou, R.; Zhang, X. Glycolysis: A Fork in the Path of Normal and Pathological Pregnancy. FASEB J. 2023, 37, e23263. [Google Scholar] [CrossRef]
- Gu, W.; Gaeta, X.; Sahakyan, A.; Chan, A.B.; Hong, C.P.; Kim, R.; Braas, D.; Plath, K.; Lowry, W.E.; Christofk, H.R. Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell 2016, 19, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Murakami, Y.; Shigeta, D.; Tomosugi, M.; Sakata-Haga, H.; Hatta, T.; Shoji, H. Glycolytic Activity Is Required for the Onset of Neural Plate Folding during Neural Tube Closure in Mouse Embryos. Front. Cell Dev. Biol. 2023, 11, 1212375. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Nishida, Y.; Harada, E.; Sakai, K.; Narahara, H. GC-MS/MS Analysis of Metabolites Derived from a Single Human Blastocyst. Metabolomics 2021, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Eldarov, C.; Gamisonia, A.; Chagovets, V.; Ibragimova, L.; Yarigina, S.; Smolnikova, V.; Kalinina, E.; Makarova, N.; Zgoda, V.; Sukhikh, G.; et al. LC-MS Analysis Revealed the Significantly Different Metabolic Profiles in Spent Culture Media of Human Embryos with Distinct Morphology, Karyotype and Implantation Outcomes. Int. J. Mol. Sci. 2022, 23, 2706. [Google Scholar] [CrossRef] [PubMed]
- Cheredath, A.; Uppangala, S.; Asha, C.S.; Jijo, A.; Vani Lakshmi, R.; Kumar, P.; Joseph, D.; Nagana Gowda, G.A.; Kalthur, G.; Adiga, S.K. Combining Machine Learning with Metabolomic and Embryologic Data Improves Embryo Implantation Prediction. Reprod. Sci. 2023, 30, 984–994. [Google Scholar] [CrossRef]
- Krisher, R.L.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Omics as a Window to View Embryo Viability. Fertil. Steril. 2015, 103, 333–341. [Google Scholar] [CrossRef]
- Weng, L. IVF-on-a-Chip: Recent Advances in Microfluidics Technology for In Vitro Fertilization. SLAS Technol. 2019, 24, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal Intake of Methyl-Group Donors Affects DNA Methylation of Metabolic Genes in Infants. Clin. Epigenetics 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, L.; Kempe, H.; Verschure, P.J. Epigenetic Imprinting during Assisted Reproductive Technologies: The Effect of Temporal and Cumulative Fluctuations in Methionine Cycling on the DNA Methylation State. Mol. Reprod. Dev. 2016, 83, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Clément, P.; Dale, B. DNA Methylation Patterns in the Early Human Embryo and the Epigenetic/Imprinting Problems: A Plea for a More Careful Approach to Human Assisted Reproductive Technology (ART). Int. J. Mol. Sci. 2019, 20, E1342. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Afeiche, M.C.; Wright, D.L.; Toth, T.L.; Williams, P.L.; Gillman, M.W.; Hauser, R.; Chavarro, J.E. Dietary Folate and Reproductive Success among Women Undergoing Assisted Reproduction. Obstet. Gynecol. 2014, 124, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C. One Carbon Metabolism in Pregnancy: Impact on Maternal, Fetal and Neonatal Health. Mol. Cell Endocrinol. 2016, 435, 48–60. [Google Scholar] [CrossRef]
- Siristatidis, C.S.; Sertedaki, E.; Vaidakis, D.; Varounis, C.; Trivella, M. Metabolomics for Improving Pregnancy Outcomes in Women Undergoing Assisted Reproductive Technologies. Cochrane Database Syst. Rev. 2018, 2018, CD011872. [Google Scholar] [CrossRef]

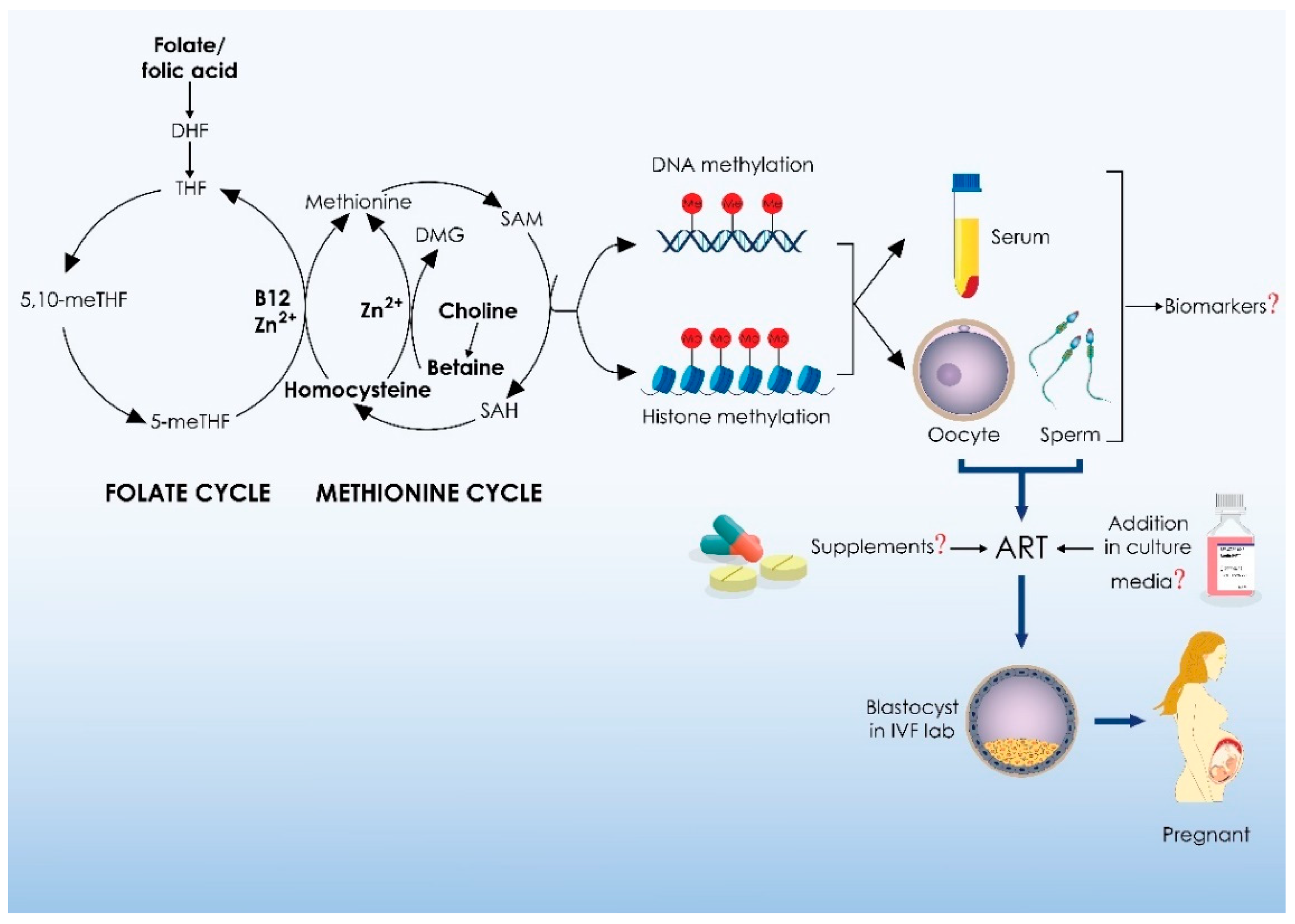
| Study | Intervention/Observation | Outcome Measure | Results |
|---|---|---|---|
| Komiya et al., 2023 [210] | Zinc serum levels in males | Total motile sperm count | NS |
| Dadgar et al., 2022 [211] | Zinc supplementation in males | Semen parameters and Sperm DNA fragmentation | Increased normal spermatozoa morphology and decreased DNA fragmentation index following zinc supplementation |
| Chabchoub et al., 2021 [212] | Zinc serum levels in males | Zinc levels in fertile vs. infertile men | Higher in fertile men (cut-off value: 111.8 µg/dL, AUC: 0.928) |
| Wang et al., 2021 [213] | Zinc serum levels in females | Adverse in vitro fertilization outcomes (failure to achieve clinical pregnancy) | Lower zinc levels were associated with lower clinical pregnancy rates |
| Schisterman et al., 2020 [214] | Zinc and folic acid supplementation in males | Multiple reproductive outcomes | NS |
| Tulic et al., 2019 [215] | Zinc serum levels in females | Deliveries and miscarriages | NS |
| Wdowiak et al., 2018 [216] | Zinc follicular fluid levels | Clinical pregnancy | NS |
| Berkovitz et al., 2018 [217] | Addition of zinc in cryoprotection media | Semen parameters | Statistical analysis was not performed |
| Ingle et al., 2017 [218] | Follicular fluid and urine zinc levels | Multiple reproductive outcomes | Negative correlation between follicular fluid zinc levels and fertilization rate; Positive correlation between urine zinc levels and number of available embryos |
| Isaac et al., 2017 [219] | Addition of zinc oxide nanoparticles in cryoprotection media | Post-thaw semen parameters and chromatin integrity | Lower chromatin damage following addition of zinc oxide nanoparticles in cryoprotectant media |
| Giacone et al., 2017 [220] | Addition of zinc, D-aspartic acid, and coenzyme Q10 in sperm culture media | Progressive motility following 3 h of incubation and swim-up | Addition of zinc, D-aspartic acid, and coenzyme Q10 to sperm culture media following 3 h incubation resulted in increased progressive motility in asthenozoospermic men; following swim-up increased progressive motility in all samples |
| Nematollahi-Mahani et al., 2014 [221] | Zinc sulfate supplementation and zinc sulfate plus folic acid supplementation following varicocelectomy | Sperm parameters | Zinc sulfate supplementation increased sperm normal morphology; zinc sulfate plus folic acid supplementation increases sperm concentration, progressive motility, and normal morphology |
| Singh et al., 2013 [222] | Follicular fluid zinc levels | Endometriosis vs. tubal factor infertility; pregnancy within groups | Lower level of zinc in endometriosis group; higher levels of zinc in women with endometriosis who achieved pregnancy |
| Kotdawala et al., 2012 [223] | Addition of zinc in cryoprotection media | Post-thaw sperm parameters and chromatin integrity | Increased progressive motility and chromatin integrity following addition of zinc |
| Atig et al., 2012 [224] | Seminal plasma zinc levels | Sperm parameters | Positive correlation with sperm concentration and motility; negative correlation with normal morphology |
| Dickerson et al., 2011 [225] | Hair and serum zinc levels in females | Follicle number and oocyte yield | Positive correlation of hair zinc levels with oocyte yield; no correlation between hair and serum zinc levels |
| Colagar et al., 2009 [226] | Seminal plasma zinc levels | Zinc levels in fertile vs. infertile men | Fertile men presented with higher zinc levels compared to those infertile |
| Omu et al., 2008 [227] | Zinc supplementation alone or in combination with other vitamins in males | Semen parameters and DNA fragmentation index | Zinc supplementation alone or in combination with other vitamins increased sperm parameters and decreased DNA fragmentation index |
| Ebisch et al., 2006 [228] | Zinc supplementation in males | Semen parameters and reproductive hormone levels | Zinc supplementation increased sperm concentration |
| Benoff et al., 1999 [229] | Seminal plasma zinc levels | Semen parameters; fertilization and clinical pregnancy rates | NS |
| Tikkiwal et al., 1987 [230] | Supplementation of zinc in males | Semen parameters | Increase in sperm count, progressive motility and normal morphology |
| Study | Intervention/Observation | Outcome Measure | Results |
|---|---|---|---|
| De Cosmi et al., 2023 [257] | Serum folate levels in females | Multiple IVF outcomes | NS |
| Polzikov et al., 2022 [256] | Serum folate levels in females (comparison between highest and lowest quantiles of folate concentration) | Number of oocytes retrieved, clinical pregnancy, and live birth | Women in the highest quantile presented with decreased oocyte yield and decreased odds for clinical pregnancy and live birth |
| Tabatabaie et al., 2022 [258] | Supplementation of folate and folate with myoinositol in polycystic ovarian syndrome cases | Number of oocytes retrieved, MII rate, fertilization rate, and embryo quality | Only women receiving myoinositol and folate presented with increased oocyte yield, MII and fertilization rate, and embryo quality compared to both other groups |
| D’Argent et al., 2021 [259] | Supplementation of folate in males | Sperm parameters, sperm DNA fragmentation, positive hCG, and clinical pregnancy | Decrease in sperm DNA fragmentation, increase in positive hCG rate. NS in sperm parameters and clinical pregnancy rate |
| Mohammadi et al., 2021 [260] | Supplementation of folate and folate with myoinositol in poor ovarian response cases | Number of oocytes retrieved, MII rate, fertilization rate, embryo quality, clinical pregnancy, and live-birth | NS |
| So et al., 2020 [261] | Supplementation of folate with L-arginine in women | Positive human chorionic gonadotropin test and clinical pregnancy | NS (statistical significance was observed in subgroup analysis, albeit with significantly wide confidence intervals) |
| Nazari et al., 2020 [262] | Supplementation of folate with myoinositol in women | Number of retrieved oocytes, embryo quality, fertilization, implantation, and ongoing pregnancy rates | Increased fertilization, good-quality embryo, implantation, and ongoing pregnancy rates |
| Regidor et al., 2018 [263] | Supplementation of folate with myoinositol in women | Number of retrieved oocytes, embryo quality, and fertilization rates | Improved fertilization and embryo quality rates |
| Nouri et al., 2017 [264] | Supplementation of folate among other nutrients | Embryo quality on day 3 and clinical pregnancy rate | Improved embryo quality on day 3 |
| Murto et al., 2014 [265] | Supplementation of folate in women | Clinical pregnancy | NS |
| Study | Intervention/Observation | Outcome Measure | Results |
|---|---|---|---|
| Manzur et al., 2023 [309] | Homocysteine and B12 serum levels in females | Number of embryos available for transfer | Homocysteine levels were negatively associated with number of embryos available for transfer |
| Wang et al., 2022 [310] | Homocysteine serum levels in poor ovarian response cases | Embryo quality | Negative association between homocysteine levels and embryo quality |
| Chen et al., 2021 [311] | Homocysteine serum levels in females | Clinical pregnancy | Negative association between homocysteine levels and clinical pregnancy rates |
| Razi et al., 2021 [307] | Homocysteine follicular fluid levels | Oocyte maturity, embryo quality, and clinical pregnancy | Negative association between homocysteine levels and oocyte maturity, and embryo quality and clinical pregnancy rates |
| Liu et al., 2020 [312] | Homocysteine serum levels in females | Multiple reproductive outcomes | Only negative association with number of oocytes retrieved |
| Berker et al., 2009 [302] | Homocysteine follicular fluid levels | Multiple reproductive outcomes | Negative association with fertilization rate and embryo quality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfakianoudis, K.; Zikopoulos, A.; Grigoriadis, S.; Seretis, N.; Maziotis, E.; Anifandis, G.; Xystra, P.; Kostoulas, C.; Giougli, U.; Pantos, K.; et al. The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4977. https://doi.org/10.3390/ijms25094977
Sfakianoudis K, Zikopoulos A, Grigoriadis S, Seretis N, Maziotis E, Anifandis G, Xystra P, Kostoulas C, Giougli U, Pantos K, et al. The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature. International Journal of Molecular Sciences. 2024; 25(9):4977. https://doi.org/10.3390/ijms25094977
Chicago/Turabian StyleSfakianoudis, Konstantinos, Athanasios Zikopoulos, Sokratis Grigoriadis, Nikolaos Seretis, Evangelos Maziotis, George Anifandis, Paraskevi Xystra, Charilaos Kostoulas, Urania Giougli, Konstantinos Pantos, and et al. 2024. "The Role of One-Carbon Metabolism and Methyl Donors in Medically Assisted Reproduction: A Narrative Review of the Literature" International Journal of Molecular Sciences 25, no. 9: 4977. https://doi.org/10.3390/ijms25094977






