(E)-1-(5-(Hydroxymethyl) furan-2-yl)-4,4-dimethylpent-1-en-3-one
Abstract
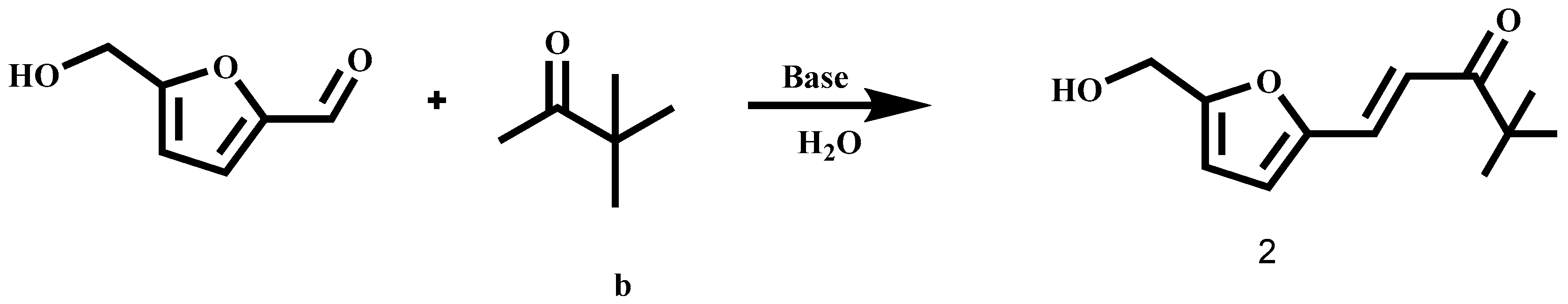
:1. Introduction
2. Results and Discussion
Chemistry
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, X.L.; Ma, Y.; Li, Y.D. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Rashid, T.; Sher, F.; Khan, A.S.; Khalid, U.; Rasheed, T.; Iqbal, H.M.N.; Murugesan, T. Effect of protic ionic liquid treatment on the pyrolysis products of lignin extracted from oil palm biomass. Fuel 2021, 291, 120133. [Google Scholar] [CrossRef]
- Fan, G.; Wang, Y.; Hu, Z.; Yan, J.; Li, J.; Song, G. Synthesis of 5-hydroxymethyl furfural from cellulose via a two-step process in polar aprotic solvent. Carbohydr. Polym. 2018, 200, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Zhao, D.D.; Guo, N.; Xue, C.H.; Mao, X.Z. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.D.; Xiang, N.; Zhu, X.; Shuang, E.; Sheng, K.C.; Zhang, X.M. Selective 5-hydroxymethylfurfural production from cellulose formate in DMSO-H2O media. Appl. Catal. B Environ. 2021, 285, 119799. [Google Scholar] [CrossRef]
- Noviany, N.; Osman, H.; Mohamad, S.; Hadi, S.; Satria, H.; Buhani, B. Synthesis of some chalcones derivatives series and their antituberculosis activity. Pure Appl. Chem. 2024, 18. [Google Scholar] [CrossRef]
- Pina, V.; da Costa Duarte, R.; Vesga-Hernández, C.; dos Santos Carvalho, R.; Melo, D.G.; Pedrozo-Penafiel, M.J.; Barreto, A.R.J.; dos Santos, A.M.; Dal-Bó, A.G.; Aucélio, R.Q.; et al. Carboxy-substituted D-z-A arylated chalcones: Synthesis, photophysical properties and preliminary evaluation as photosensitizers for DSSCs. Opt. Mater. 2024, 149, 115039. [Google Scholar] [CrossRef]
- Mahdi, I.S.; Abdula, A.M.; Jassim, A.M.N.; Baqi, Y. Design, Synthesis, Antimicrobial Properties, and Molecular Docking of Novel Furan-Derived Chalcones and Their 3,5-Diaryl-∆2-pyrazoline Derivatives. Antibiotics 2024, 13, 21. [Google Scholar] [CrossRef]
- Dandawate, P.; Ahmed, K.; Padhye, S.; Ahmad, A.; Biersack, B. Anticancer Active Heterocyclic Chalcones: Recent Developments. Anti-Cancer Agents Med. Chem. 2021, 21, 558–566. [Google Scholar] [CrossRef]
- Luo, Z.C.; Kong, J.C.; Ma, B.; Wang, Z.C.; Huang, J.; Zhao, C. Liquefaction and Hydrodeoxygenation of Polymeric Lignin Using a Hierarchical Ni Microreactor Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 2158–2166. [Google Scholar] [CrossRef]
- Su, H.; Wang, J.K.; Yan, L.F. Homogeneously Synchronous Degradation of Chitin into Carbon Dots and Organic Acids in Aqueous Solution. ACS Sustain. Chem. Eng. 2019, 7, 18476–18482. [Google Scholar] [CrossRef]
- Goswami, S.R.; Mukherjee, A.; Dumont, M.J.; Raghavan, V. One-Pot Conversion of Corn Starch into 5-Hydroxymethylfurfural in Water-Bmim Cl/MIBK Biphasic Media. Energy Fuels 2016, 30, 8349–8356. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Tajiri, R.; Mihata, A.; Yamamoto, K.; Kadokawa, J. Facile preparation of chitin gels with calcium bromide dihydrate/methanol media and their efficient conversion into porous chitins. RSC Adv. 2014, 4, 5542–5546. [Google Scholar] [CrossRef]
- Zhang, J.G.; Yan, N. Formic acid-mediated liquefaction of chitin. Green Chem. 2016, 18, 5050–5058. [Google Scholar] [CrossRef]
- Kang, S.M.; Fu, J.X.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Gu, L.J.; Li, C.Y.; Niu, X.Y.; Liu, X.L.; Bu, Z.W.; Wang, Q.L. Organophosphine as an Alkyl Transfer Shuttle for the Direct β-Alkylation of Chalcones Using Alkyl Halides. J. Org. Chem. 2023, 88, 534–539. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, L.; He, P.; Qin, Y. (E)-1-(5-(Hydroxymethyl) furan-2-yl)-4,4-dimethylpent-1-en-3-one. Molbank 2024, 2024, M1818. https://doi.org/10.3390/M1818
Wang Z, Zhou L, He P, Qin Y. (E)-1-(5-(Hydroxymethyl) furan-2-yl)-4,4-dimethylpent-1-en-3-one. Molbank. 2024; 2024(2):M1818. https://doi.org/10.3390/M1818
Chicago/Turabian StyleWang, Zhongwei, Luxiao Zhou, Peng He, and Yukun Qin. 2024. "(E)-1-(5-(Hydroxymethyl) furan-2-yl)-4,4-dimethylpent-1-en-3-one" Molbank 2024, no. 2: M1818. https://doi.org/10.3390/M1818



