Atomistic Details of Methyl Linoleate Pyrolysis: Direct Molecular Dynamics Simulation of Converting Biodiesel to Petroleum Products
Abstract
:1. Introduction
2. Methods
2.1. Electronic Structure Calculations and Model for the Potential Energy Surface
2.2. Computational Details of the Direct Dynamics Simulations
3. Results and Discussion
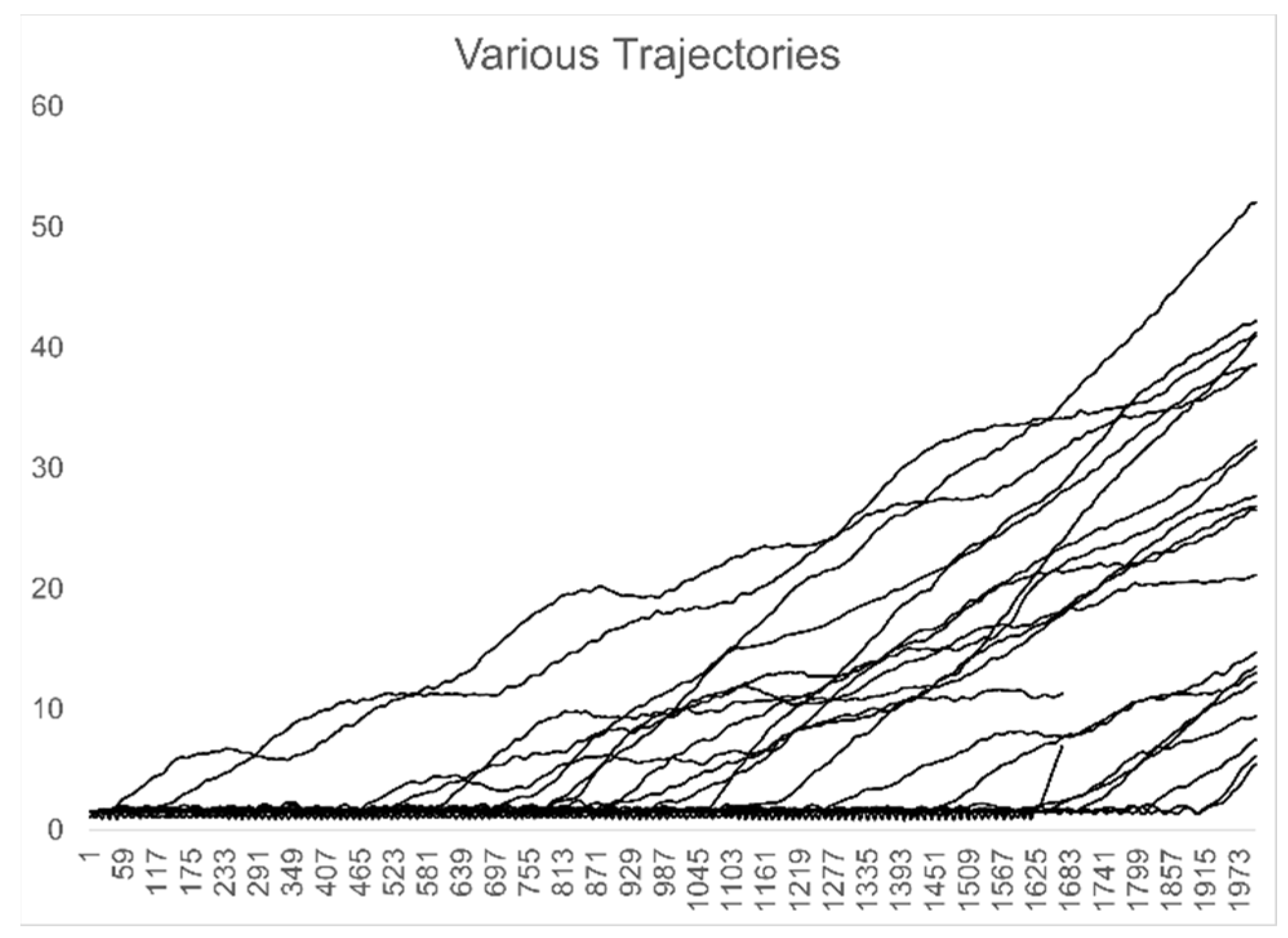
3.1. Ensemble Analysis
3.2. Homology of Hydrocarbon Products
3.3. Ratio of CO2 to CO Production
3.4. Alignment of Ensemble Results and Bond-Dissociation Energy (BDE) Analysis
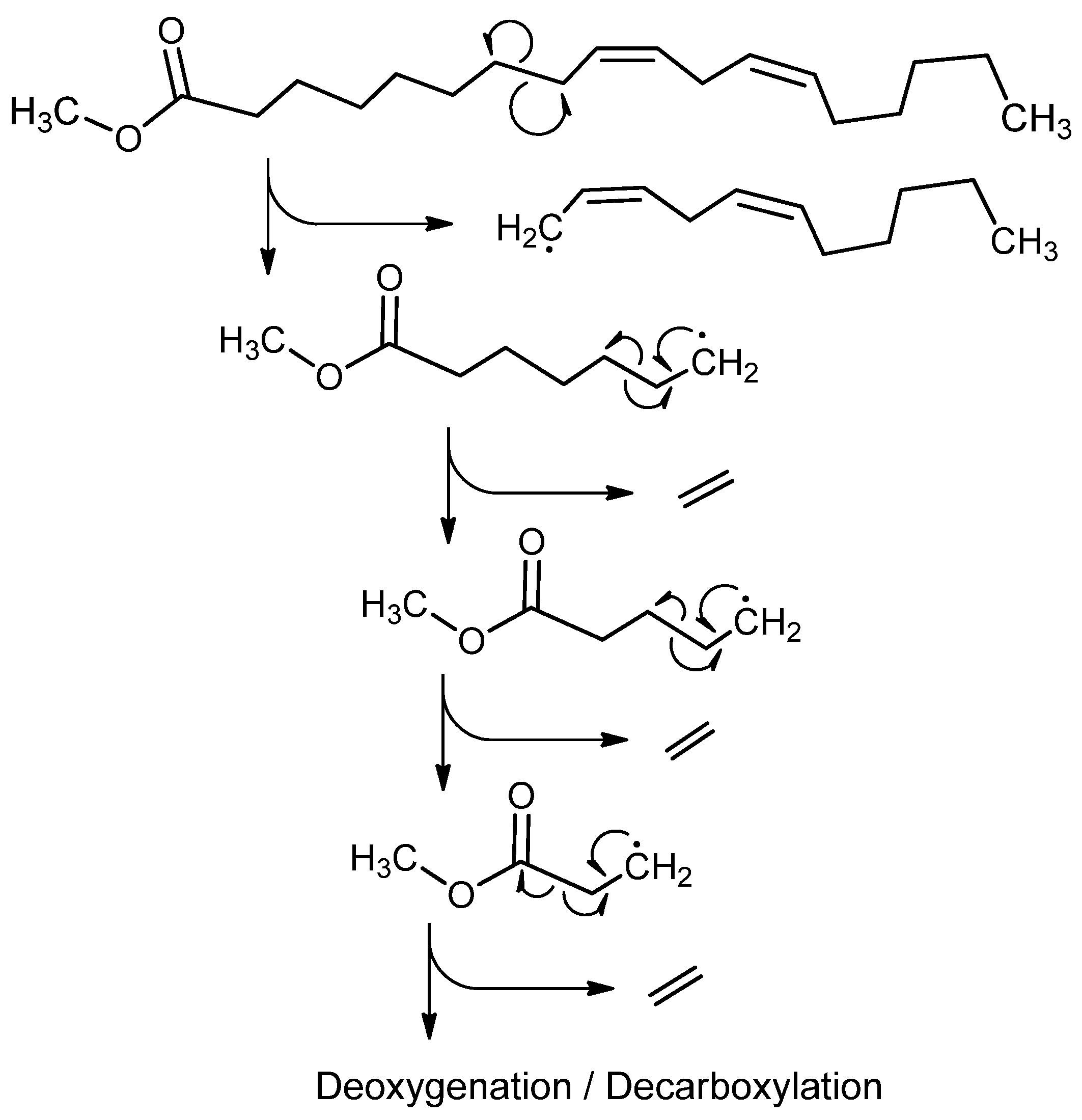
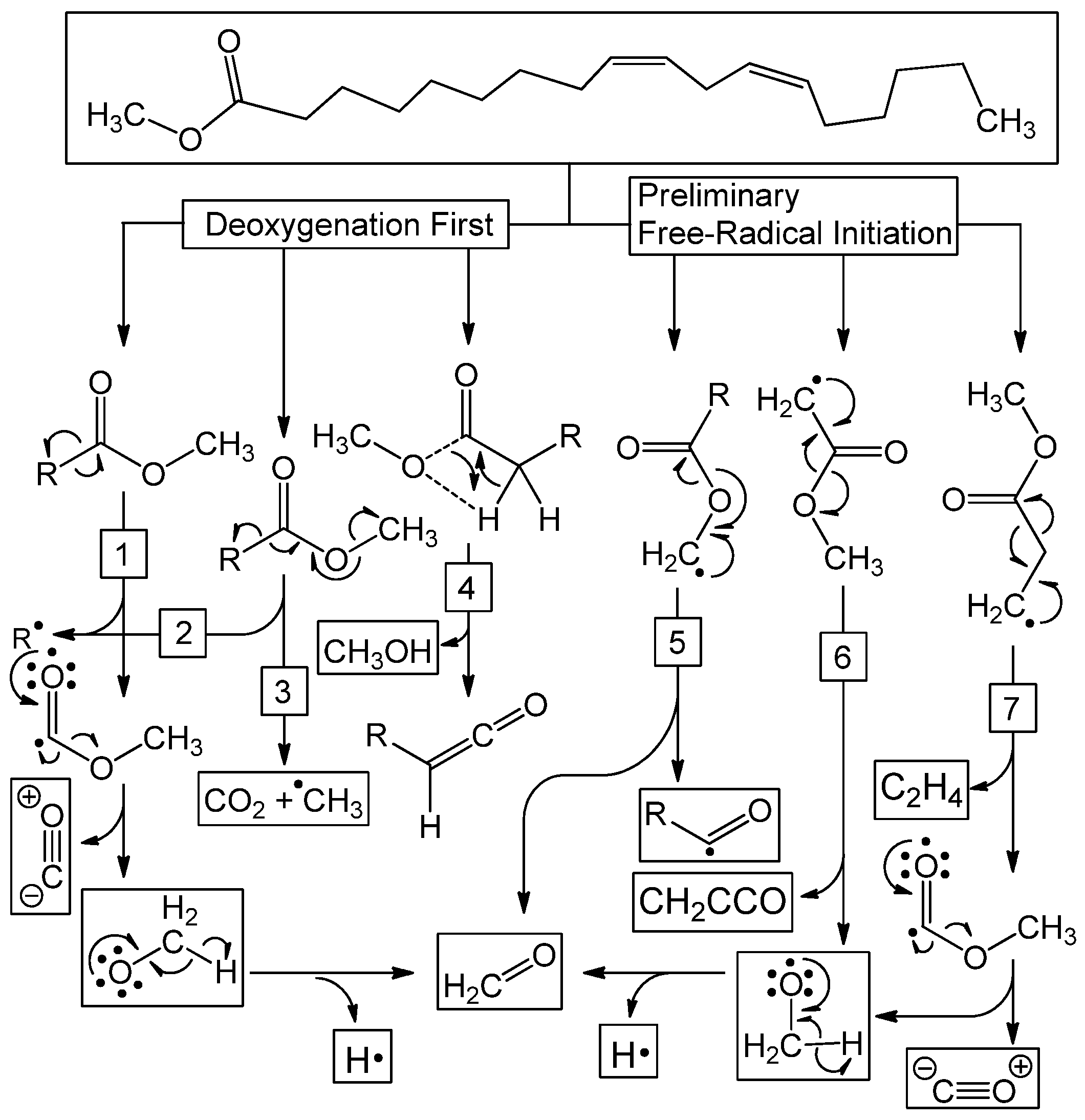
3.5. Atomic-Level Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hess, J.; Bednarz, D.; Bae, J.; Pierce, J. Petroleum and Health Care: Evaluating and Managing Health Care’s Vulnerability to Petroleum Supply Shifts. Am. J. Public Health 2011, 101, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Owusu, P.A.; Asumadu-Sarkodie, S. A Review of Renewable Energy Sources, Sustainability Issues and Climate Change Mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Al-Sharrah, G.; Elkamel, A.; Almanssoor, A. Sustainability Indicators for Decision-Making and Optimisation in the Process Industry: The Case of the Petrochemical Industry. Chem. Eng. Sci. 2010, 65, 1452–1461. [Google Scholar] [CrossRef]
- Candeia, R.A.; Silva, M.C.D.; Carvalho Filho, J.R.; Brasilino, M.G.A.; Bicudo, T.C.; Santos, I.M.G.; Souza, A.G. Influence of Soybean Biodiesel Content on Basic Properties of Biodiesel–Diesel Blends. Fuel 2009, 88, 738–743. [Google Scholar] [CrossRef]
- Namazi, H. Polymers in Our Daily Life. Bioimpacts 2017, 7, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Jasinska-Walc, L.; Bouyahyi, M.; Duchateau, R. Potential of Functionalized Polyolefins in a Sustainable Polymer Economy: Synthetic Strategies and Applications. Acc. Chem. Res. 2022, 55, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Koczoń, P.; Bartyzel, B.; Iuliano, A.; Klensporf-Pawlik, D.; Kowalska, D.; Majewska, E.; Tarnowska, K.; Zieniuk, B.; Gruczyńska-Sękowska, E. Chemical Structures, Properties, and Applications of Selected Crude Oil-Based and Bio-Based Polymers. Polymers 2022, 14, 5551. [Google Scholar] [CrossRef]
- Bouaid, A.; El boulifi, N.; Hahati, K.; Martinez, M.; Aracil, J. Biodiesel Production from Biobutanol. Improvement of Cold Flow Properties. Chem. Eng. J. 2014, 238, 234–241. [Google Scholar] [CrossRef]
- Aboim, J.B.; Oliveira, D.; Ferreira, J.E.; Siqueira, A.S.; Dall’Agnol, L.T.; Rocha Filho, G.N.; Gonçalves, E.C.; Nascimento, L.A. Determination of Biodiesel Properties Based on a Fatty Acid Profile of Eight Amazon Cyanobacterial Strains Grown in Two Different Culture Media. RSC Adv. 2016, 6, 109751–109758. [Google Scholar] [CrossRef]
- Lappi, H.; Alén, R. Production of Vegetable Oil-Based Biofuels—Thermochemical Behavior of Fatty Acid Sodium Salts during Pyrolysis. J. Anal. Appl. Pyrolysis 2009, 86, 274–280. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Mofijur, M.; Mahlia, T.M.I.; Ok, Y.S. Pyrolysis of Waste Oils for the Production of Biofuels: A Critical Review. J. Hazard. Mater. 2022, 424, 127396. [Google Scholar] [CrossRef] [PubMed]
- Graboski, M.S.; McCormick, R.L. Combustion of Fat and Vegetable Oil Derived Fuels in Diesel Engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Lin, L.; Cunshan, Z.; Vittayapadung, S.; Xiangqian, S.; Mingdong, D. Opportunities and Challenges for Biodiesel Fuel. Appl. Energy 2011, 88, 1020–1031. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Parvizsedghy, R.; Sadrameli, S.M.; Towfighi Darian, J. Upgraded Biofuel Diesel Production by Thermal Cracking of Castor Biodiesel. Energy Fuels 2016, 30, 326–333. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Knothe, G. Improving Biodiesel Fuel Properties by Modifying Fatty Ester Composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mohamed, M.; Tan, C.-K.; Fouda, A.; Gad, M.S.; Abu-Elyazeed, O.; Hashem, A.-F. Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil. Energies 2020, 13, 5708. [Google Scholar] [CrossRef]
- Wilson, Z.R.; Siebert, M.R. Methyl Linoleate and Methyl Oleate Bond Dissociation Energies: Electronic Structure Fishing for Wise Crack Products. Energy Fuels 2018, 32, 1779–1787. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Li, Y.; Ye, Y.; Tian, J.; Li, J.; Xu, Y.; Lv, J. Research and Optimization of Hydrogen Addition and EGR on the Combustion, Performance, and Emission of the Biodiesel-Hydrogen Dual-Fuel Engine with Different Loads Based on the RSM. Heliyon 2024, 10, e23389. [Google Scholar] [CrossRef]
- Modabberian, A.; Storm, X.; Shamekhi, A.-M.; Vasudev, A.; Zenger, K.; Hyvönen, J.; Mikulski, M. Low Temperature Combustion Modeling and Predictive Control of Marine Engines. Appl. Sci. 2024, 14, 2033. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel Production—Current State of the Art and Challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhu, Y.; Tavlarides, L.L. Effect of Thermal Decomposition on Biodiesel Viscosity and Cold Flow Property. Fuel 2014, 117, 981–988. [Google Scholar] [CrossRef]
- Clark, J.; Farmer, T.; Hunt, A.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. IJMS 2015, 16, 17101–17159. [Google Scholar] [CrossRef] [PubMed]
- Amber, S.; Kamal, M.A. Production of Hydrocarbons by Catalytic Cracking of Stearic Acid under Atmospheric Pressure for Petrochemical Replacement. Pet. Sci. Technol. 2019, 37, 146–154. [Google Scholar] [CrossRef]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Thermal Deoxygenation and Pyrolysis of Oleic Acid. J. Anal. Appl. Pyrolysis 2014, 105, 1–7. [Google Scholar] [CrossRef]
- Maher, K.D.; Kirkwood, K.M.; Gray, M.R.; Bressler, D.C. Pyrolytic Decarboxylation and Cracking of Stearic Acid. Ind. Eng. Chem. Res. 2008, 47, 5328–5336. [Google Scholar] [CrossRef]
- Yin, C. Microwave-Assisted Pyrolysis of Biomass for Liquid Biofuels Production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-Oil, Solid and Gaseous Biofuels from Biomass Pyrolysis Processes-An Overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Boocock, D.G.B.; Konar, S.K.; Glaser, G. The Formation of Petrodiesel by the Pyrolysis of Fatty Acid Methyl Esters Over Activated Alumina. In Progress in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Wiley: Hoboken, NJ, USA, 2001; pp. 1517–1524. ISBN 978-0-632-05533-3. [Google Scholar]
- Li, X.; Xu, X.; You, X.; Truhlar, D.G. Benchmark Calculations for Bond Dissociation Enthalpies of Unsaturated Methyl Esters and the Bond Dissociation Enthalpies of Methyl Linolenate. J. Phys. Chem. A 2016, 120, 4025–4036. [Google Scholar] [CrossRef]
- Pires de Oliveira, I.; Caires, A.R.L. Molecular Arrangement in Diesel/Biodiesel Blends: A Molecular Dynamics Simulation Analysis. Renew. Energy 2019, 140, 203–211. [Google Scholar] [CrossRef]
- Lin, R.; Zhu, Y.; Tavlarides, L.L. Mechanism and Kinetics of Thermal Decomposition of Biodiesel Fuel. Fuel 2013, 106, 593–604. [Google Scholar] [CrossRef]
- Asomaning, J.; Mussone, P.; Bressler, D.C. Pyrolysis of Polyunsaturated Fatty Acids. Fuel Process. Technol. 2014, 120, 89–95. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Al-Hashimi, N.; Shibl, M.F.; Yoshizawa, K.; El-Nahas, A.M. Thermochemistry and Kinetics of the Thermal Degradation of 2-Methoxyethanol as Possible Biofuel Additives. Sci. Rep. 2019, 9, 4535. [Google Scholar] [CrossRef]
- Lynch, B.J.; Zhao, Y.; Truhlar, D.G. Effectiveness of Diffuse Basis Functions for Calculating Relative Energies by Density Functional Theory. J. Phys. Chem. A 2003, 107, 1384–1388. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Steinmetz, M. Effects of London Dispersion Correction in Density Functional Theory on the Structures of Organic Molecules in the Gas Phase. Phys. Chem. Chem. Phys. 2013, 15, 16031. [Google Scholar] [CrossRef]
- Schmid, E.W.; Zieģelmann, H.; Stumpf, H. The Quantum Mechanical Three-Body Problem: Vieweg Tracts in Pure and Applied Physics; Elsevier Science: Kent, UK, 2014; ISBN 978-1-4831-6078-8. [Google Scholar]
- Wilson, Z.R. Ab Initio Methyl Linoleate Bond Dissociation Energies: First Principles Fishing for Wise Crack Products. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2017. [Google Scholar]
- Abrams, C.F.; Vanden-Eijnden, E. Large-Scale Conformational Sampling of Proteins Using Temperature-Accelerated Molecular Dynamics. Proc. Natl. Acad. Sci. USA 2010, 107, 4961–4966. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-Q.; Lapelosa, M.; Vanden-Eijnden, E.; Abrams, C.F. Full Kinetics of CO Entry, Internal Diffusion, and Exit in Myoglobin from Transition-Path Theory Simulations. J. Am. Chem. Soc. 2015, 137, 3041–3050. [Google Scholar] [CrossRef]
- Schlick, T. Molecular Dynamics-Based Approaches for Enhanced Sampling of Long-Time, Large-Scale Conformational Changes in Biomolecules. F1000 Biol. Rep. 2009, 1. [Google Scholar] [CrossRef]
- Miron, R.A.; Fichthorn, K.A. Accelerated Molecular Dynamics with the Bond-Boost Method. J. Chem. Phys. 2003, 119, 6210–6216. [Google Scholar] [CrossRef]
- Abrams, C.; Bussi, G. Enhanced Sampling in Molecular Dynamics Using Metadynamics, Replica-Exchange, and Temperature-Acceleration. Entropy 2013, 16, 163–199. [Google Scholar] [CrossRef]
- Almas, Q.L.; Keefe, B.L.; Profitt, T.; Pearson, J.K. Choosing an Appropriate Model Chemistry in a Big Data Context: Application to Dative Bonding. Comput. Theor. Chem. 2016, 1085, 46–55. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Pratihar, S.; Hase, W.L. Direct Dynamics Simulations of the CH2 + O2 Reaction on the Ground- and Excited-State Singlet Surfaces. J. Phys. Chem. A 2019, 123, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- So/rensen, M.R.; Voter, A.F. Temperature-Accelerated Dynamics for Simulation of Infrequent Events. J. Chem. Phys. 2000, 112, 9599–9606. [Google Scholar] [CrossRef]
- Savage, P.E. Mechanisms and Kinetics Models for Hydrocarbon Pyrolysis. J. Anal. Appl. Pyrolysis 2000, 54, 109–126. [Google Scholar] [CrossRef]
- Lappi, H.; Alén, R. Pyrolysis of Vegetable Oil Soaps—Palm, Olive, Rapeseed and Castor Oils. J. Anal. Appl. Pyrolysis 2011, 91, 154–158. [Google Scholar] [CrossRef]
- Krüger, S.; Gaich, T. Recent Applications of the Divinylcyclopropane–Cycloheptadiene Rearrangement in Organic Synthesis. Beilstein J. Org. Chem. 2014, 10, 163–193. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, M.J.; Siebert, M.R. Atomistic Details of Methyl Linoleate Pyrolysis: Direct Molecular Dynamics Simulation of Converting Biodiesel to Petroleum Products. Energies 2024, 17, 2433. https://doi.org/10.3390/en17102433
Bakker MJ, Siebert MR. Atomistic Details of Methyl Linoleate Pyrolysis: Direct Molecular Dynamics Simulation of Converting Biodiesel to Petroleum Products. Energies. 2024; 17(10):2433. https://doi.org/10.3390/en17102433
Chicago/Turabian StyleBakker, Michael J., and Matthew R. Siebert. 2024. "Atomistic Details of Methyl Linoleate Pyrolysis: Direct Molecular Dynamics Simulation of Converting Biodiesel to Petroleum Products" Energies 17, no. 10: 2433. https://doi.org/10.3390/en17102433






