Multifunctional Superamphiphobic Coating Based on Fluorinated TiO2 toward Effective Anti-Corrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TiO2@fluoroPOS Fillers
2.3. Preparation of P/TiO2@fluoroPOS Coatings
2.4. Characterization
3. Results and Discussion
3.1. Analyses of TiO2@fluoroPOS Filler
3.2. Surface Morphology of TiO2@fluoroPOS Fillers and P/TiO2@fluoroPOS Coatings
3.3. Wettability of P/TiO2@fluoroPOS Coatings
3.4. Corrosion Resistance of P/TiO2@fluoroPOS Coatings
3.5. Oil Repellency of P/TiO2@fluoroPOS Coatings
3.6. Anti-Icing of P/TiO2@fluoroPOS Coatings
3.7. Anti-Waxing of P/TiO2@fluoroPOS Coatings
3.8. Self-Cleaning Ability of P/TiO2@fluoroPOS Coatings
3.9. Mechanical Stability of P/TiO2@fluoroPOS Coatings
3.9.1. Water Jetting Test
3.9.2. Sandpaper Abrasion Test
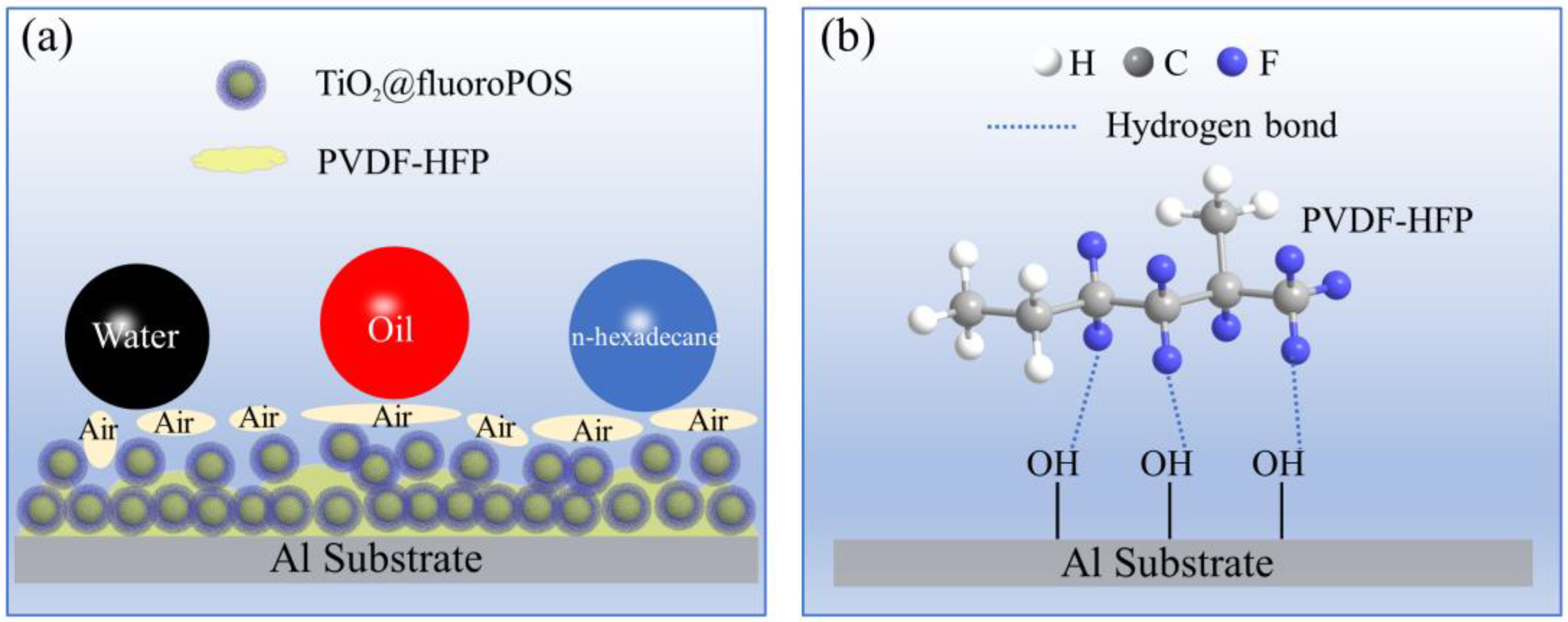
3.10. Superamphiphobicity Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, G.; Qiao, Z.; Hui, Z.; Tuo, Y.; Zheng, W.; Chen, X.; Li, S. The Waterborne Superamphiphobic Coatings with Antifouling, High Temperature Resistance, and Corrosion Resistance. ACS Omega 2023, 8, 13578–13592. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, H.; Jia, Q.; Chen, R. Preparation of a transparent coating with superamphiphobic and antifouling properties. Mater. Chem. Phys. 2023, 293, 126888. [Google Scholar] [CrossRef]
- Qiao, Z.; Ren, G.; Chen, X.; Gao, Y.; Tuo, Y.; Lu, C. Fabrication of Robust Waterborne Superamphiphobic Coatings with Antifouling, Heat Insulation, and Anticorrosion. ACS Omega 2022, 8, 804–818. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, S.; Yu, P.; Wang, B.; Mu, L.; Zhang, X.; Zhu, Y.; Wang, B.; Wang, H.; Zhu, J. Superamphiphobic and Electroactive Nanocomposite toward Self-Cleaning, Antiwear, and Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2016, 8, 12481–12493. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xu, Q.; Tong, J.; Liu, S.; Hu, Y.; Guo, Q.; Wu, H.; Li, W.; Zhao, Q.; Chen, R. Facile preparation of pliable superamphiphobic papers with high and durable liquid repellency for anti-corrosion and open surface microfluidics. Appl. Surf. Sci. 2022, 606, 154845, Erratum in Appl. Surf. Sci. 2023, 617, 155517. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Xu, W.; Duan, J.; Hou, B. Hybrid superamphiphobic anti-corrosion coating with integrated functionalities of liquid repellency, self-cleaning, and anti-icing. J. Mater. Sci. Technol. 2024, 184, 256–268. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, G.; Zhang, M.; Wang, X.; Song, Y.; Liu, S.; Cai, Y.; Wu, D.; Chu, J.; et al. Directional rebound of compound droplets on asymmetric self-grown tilted mushroom-like micropillars for anti-bacterial and anti-icing applications. Chem. Eng. J. 2023, 472, 144949. [Google Scholar] [CrossRef]
- Wei, J.; Liang, W.; Zhang, J. Preparation of Mechanically Stable Superamphiphobic Coatings via Combining Phase Separation of Adhesive and Fluorinated SiO2 for Anti-Icing. Nanomaterials 2023, 13, 1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, H.; Zhang, X. Super-robust superamphiphobic surface with anti-icing property. RSC Adv. 2019, 9, 27702–27709. [Google Scholar] [CrossRef]
- Long, Y.; Yin, X.; Mu, P.; Wang, Q.; Hu, J.; Li, J. Slippery liquid-infused porous surface (SLIPS) with superior liquid repellency, anti-corrosion, anti-icing and intensified durability for protecting substrates. Chem. Eng. J. 2020, 401, 126137. [Google Scholar] [CrossRef]
- Li, Z.; Li, K.; Li, X.; Feng, Y.; Li, H.; Wang, H. Preparation of linseed oil-loaded porous glass bubble/wax microcapsules for corrosion- and wear-resistant difunctional coatings. Chem. Eng. J. 2022, 437, 135403. [Google Scholar] [CrossRef]
- Meena Narayana Menon, D.; Giardino, M.; Janner, D. Tunable pulsewidth nanosecond laser texturing: From environment friendly superhydrophobic to superamphiphobic surfaces. Appl. Surf. Sci. 2023, 610, 155356. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N. Superhydrophobic and superamphiphobic materials for the conservation of natural stone: An overview. Constr. Build. Mater. 2022, 320, 126175. [Google Scholar] [CrossRef]
- Cao, C.; Yi, B.; Zhang, J.; Hou, C.; Wang, Z.; Lu, G.; Huang, X.; Yao, X. Sprayable superhydrophobic coating with high processibility and rapid damage-healing nature. Chem. Eng. J. 2020, 392, 124834. [Google Scholar] [CrossRef]
- Yang, Y.; He, H.; Li, Y.; Qiu, J. Using Nanoimprint Lithography to Create Robust, Buoyant, Superhydrophobic PVB/SiO2 Coatings on wood Surfaces Inspired by Red roses petal. Sci. Rep. 2019, 9, 9961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-h.; Wang, H.-j.; Liang, Y.-h.; Li, X.-j.; Ren, L.-q.; Cui, Z.-q.; Luo, C. One-step fabrication of robust superhydrophobic and superoleophilic surfaces with self-cleaning and oil/water separation function. Sci. Rep. 2018, 8, 3869. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Z.; Liu, W. Biomimetic Multi-Functional Superamphiphobic FOTS-TiO2 Particles beyond Lotus Leaf. ACS Appl. Mater. Interfaces 2016, 8, 27188–27198. [Google Scholar] [CrossRef]
- Zhou, H.; Niu, H.; Wang, H.; Lin, T. Self-Healing Superwetting Surfaces, Their Fabrications, and Properties. Chem. Rev. 2022, 123, 663–700. [Google Scholar] [CrossRef]
- Si, W.; Guo, Z. Enhancing the lifespan and durability of superamphiphobic surfaces for potential industrial applications: A review. Adv. Colloid Interface Sci. 2022, 310, 102797. [Google Scholar] [CrossRef]
- Singh, S.; Kango, S.; Sharma, N.; Verma, R. Recent advances in the mechanical durability of superamphiphobic surfaces: A review. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 2474–2499. [Google Scholar] [CrossRef]
- Bao, M.; Tie, L.; Li, J. Smart superamphiphobic surface manipulating wetting behaviors of oil droplet in water. Tribol. Int. 2023, 179, 108189. [Google Scholar] [CrossRef]
- Hegner, K.I.; Hinduja, C.; Butt, H.-J.; Vollmer, D. Fluorine-Free Super-Liquid-Repellent Surfaces: Pushing the Limits of PDMS. Nano Lett. 2023, 23, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, J. Biomimetic Super Anti-Wetting Coatings from Natural Materials: Superamphiphobic Coatings Based on Nanoclays. Sci. Rep. 2018, 8, 12062. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, P.; Wang, M.; Wang, J.; Shi, Y.; Pan, L. Fabrication of superamphiphobic surface with re-entrant structures via self-assembly colloidal template-assisted electrochemical deposition. Surf. Interfaces 2023, 40, 103033. [Google Scholar] [CrossRef]
- Vu, H.H.; Nguyen, N.T.; Kashaninejad, N. Re-Entrant Microstructures for Robust Liquid Repellent Surfaces. Adv. Mater. Technol. 2023, 8, 2201836. [Google Scholar] [CrossRef]
- Kang, S.M.; Choi, J.S. Selective Liquid Sliding Surfaces with Springtail-Inspired Concave Mushroom-Like Micropillar Arrays. Small 2019, 16, 1904612. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.A.; Dinachali, S.S.; Nair, A.S.; Ramakrishna, S. Robust Superamphiphobic Film from Electrospun TiO2 Nanostructures. ACS Appl. Mater. Interfaces 2013, 5, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Abu Jarad, N.; Imran, H.; Imani, S.M.; Didar, T.F.; Soleymani, L. Fabrication of Superamphiphobic Surfaces via Spray Coating; a Review. Adv. Mater. Technol. 2022, 7, 2101702. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, L.; Ge, F.; Wang, G.; Zeng, Z. Robust and transparent superamphiphobic coating prepared via layer-by-layer spraying. Surf. Coat. Technol. 2021, 426, 127793. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Xia, D.; Huang, Y.; Zhao, X.; Zhang, J. Spray coated superamphiphobic surface with hot water repellency and durable corrosion resistance. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124750. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Zhang, W.; Zhang, J.; Li, J. Robust, self-cleaning, anti-fouling, superamphiphobic soy protein isolate composite films using spray-coating technique with fluorinated HNTs/SiO2. Compos. Part B Eng. 2019, 174, 107002. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Liu, W.; Steffen, W.; Butt, H.J. Fabrication of Stretchable Superamphiphobic Surfaces with Deformation-Induced Rearrangeable Structures. Adv. Mater. 2022, 34, 2107901. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Wu, J.; Guo, Z. A simple preparation of a F-TiO2-HNT superamphiphobic surface with a tube-point-like micro/nano hierarchical structure for self-cleaning and anti-fouling. New J. Chem. 2023, 47, 9989–9993. [Google Scholar] [CrossRef]
- Wang, T.; Lv, C.; Ji, L.; He, X.; Wang, S. Designing Re-Entrant Geometry: Construction of a Superamphiphobic Surface with Large-Sized Particles. ACS Appl. Mater. Interfaces 2020, 12, 49155–49164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, S.; Zhang, C.; Wang, X.-Y.; Li, Y.-L.; Jiang, Y. Facile fabrication of durable superamphiphobic PET fabrics. J. Coat. Technol. Res. 2019, 17, 711–718. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Huang, K.; Zou, H.; Dengguang, Y.; Li, Y.; Qiu, B.; Wang, X. Durable superamphiphobic nano-silica/epoxy composite coating via coaxial electrospraying method. Appl. Surf. Sci. 2018, 436, 283–292. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, S.; Geng, H.; Zhang, X.; Zhang, M.; Xu, F.; Lin, D.; Gao, Y.; Wang, H. Robust and multifunctional superamphiphobic coating toward effective anti-adhesion. Chem. Eng. J. 2022, 428, 131162. [Google Scholar] [CrossRef]
- Kuna, J.J.; Voïtchovsky, K.; Singh, C.; Jiang, H.; Mwenifumbo, S.; Ghorai, P.K.; Stevens, M.M.; Glotzer, S.C.; Stellacci, F. The effect of nanometre-scale structure on interfacial energy. Nat. Mater. 2009, 8, 837–842. [Google Scholar] [CrossRef]
- Masoud Emarati, S.; Mozammel, M. Theoretical, fundamental and experimental study of Liquid-repellency and corrosion resistance of fabricated superamphiphobic surface on Al alloy 2024. Chem. Eng. J. 2020, 387, 124046. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Milionis, A.; Bayer, I.S.; Loth, E. Recent advances in oil-repellent surfaces. Int. Mater. Rev. 2016, 61, 101–126. [Google Scholar] [CrossRef]










| Concentration of Particles (g/m2) | Water Contact Angle (°) | Standard Deviation | Oil Contact Angle (°) | Standard Deviation |
|---|---|---|---|---|
| 6.19 | 154.4 | 1.30115 | 136.3 | 2.15244 |
| 11.75 | 157.6 | 1.69794 | 144.1 | 2.61381 |
| 20.69 | 159.5 | 1.39104 | 149.8 | 1.56301 |
| 29.97 | 160.2 | 1.01833 | 154.8 | 1.57575 |
| 36.36 | 160.7 | 1.23369 | 157.2 | 1.10317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Gao, X.; Wang, X.; Shang, H.; Zhou, S. Multifunctional Superamphiphobic Coating Based on Fluorinated TiO2 toward Effective Anti-Corrosion. Materials 2024, 17, 2203. https://doi.org/10.3390/ma17102203
Huang X, Gao X, Wang X, Shang H, Zhou S. Multifunctional Superamphiphobic Coating Based on Fluorinated TiO2 toward Effective Anti-Corrosion. Materials. 2024; 17(10):2203. https://doi.org/10.3390/ma17102203
Chicago/Turabian StyleHuang, Xiao, Xinghua Gao, Xin Wang, Hongfei Shang, and Shujun Zhou. 2024. "Multifunctional Superamphiphobic Coating Based on Fluorinated TiO2 toward Effective Anti-Corrosion" Materials 17, no. 10: 2203. https://doi.org/10.3390/ma17102203






