Prediction of the Potential Distribution of Teinopalpus aureus Mell, 1923 (Lepidoptera, Papilionidae) in China Using Habitat Suitability Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Location Data
2.2. Acquisition and Selection of Bioclimate Variables
2.3. Model Building and Testing
2.4. Data Processing
3. Results
3.1. Evaluation of Model Prediction Accuracy
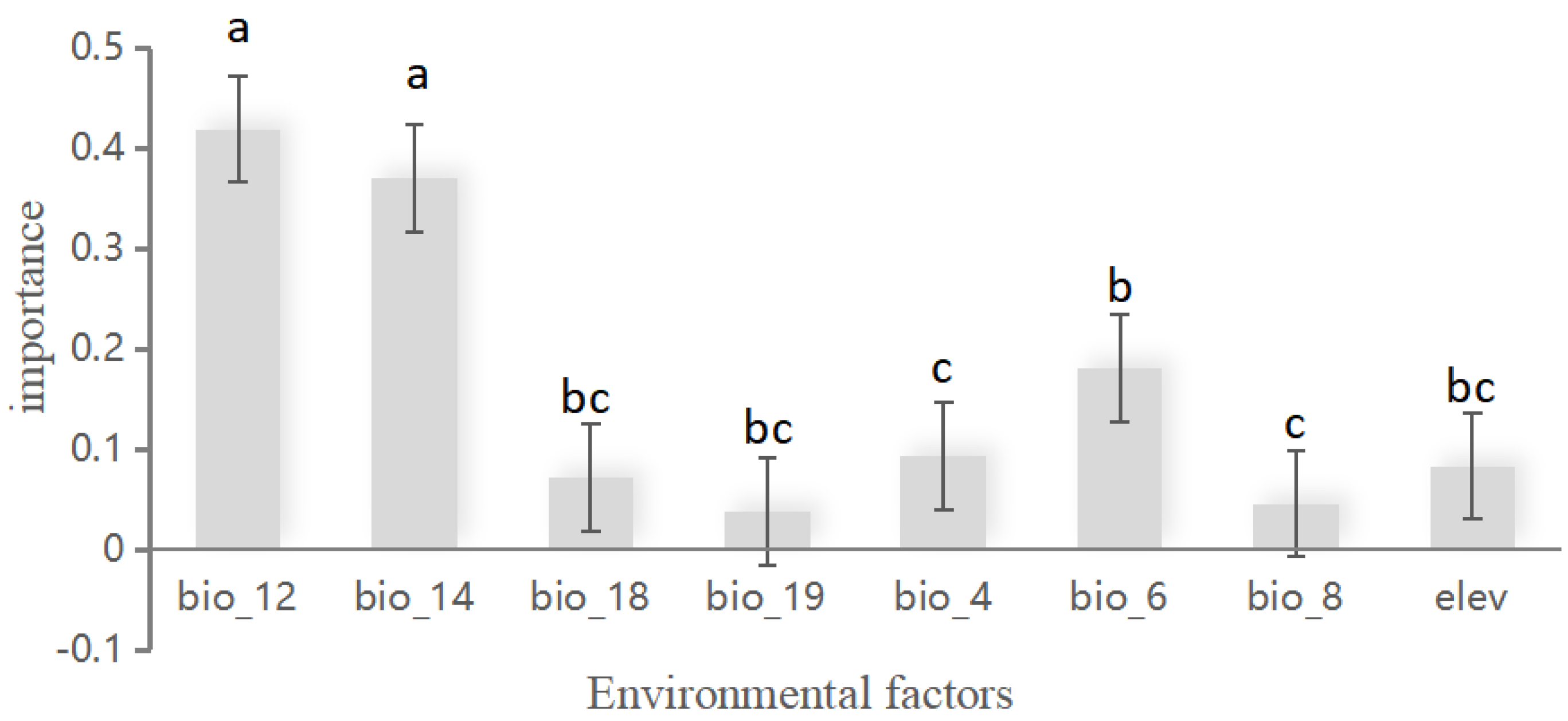
3.2. Importance Assessment of Bioclimatic Variables
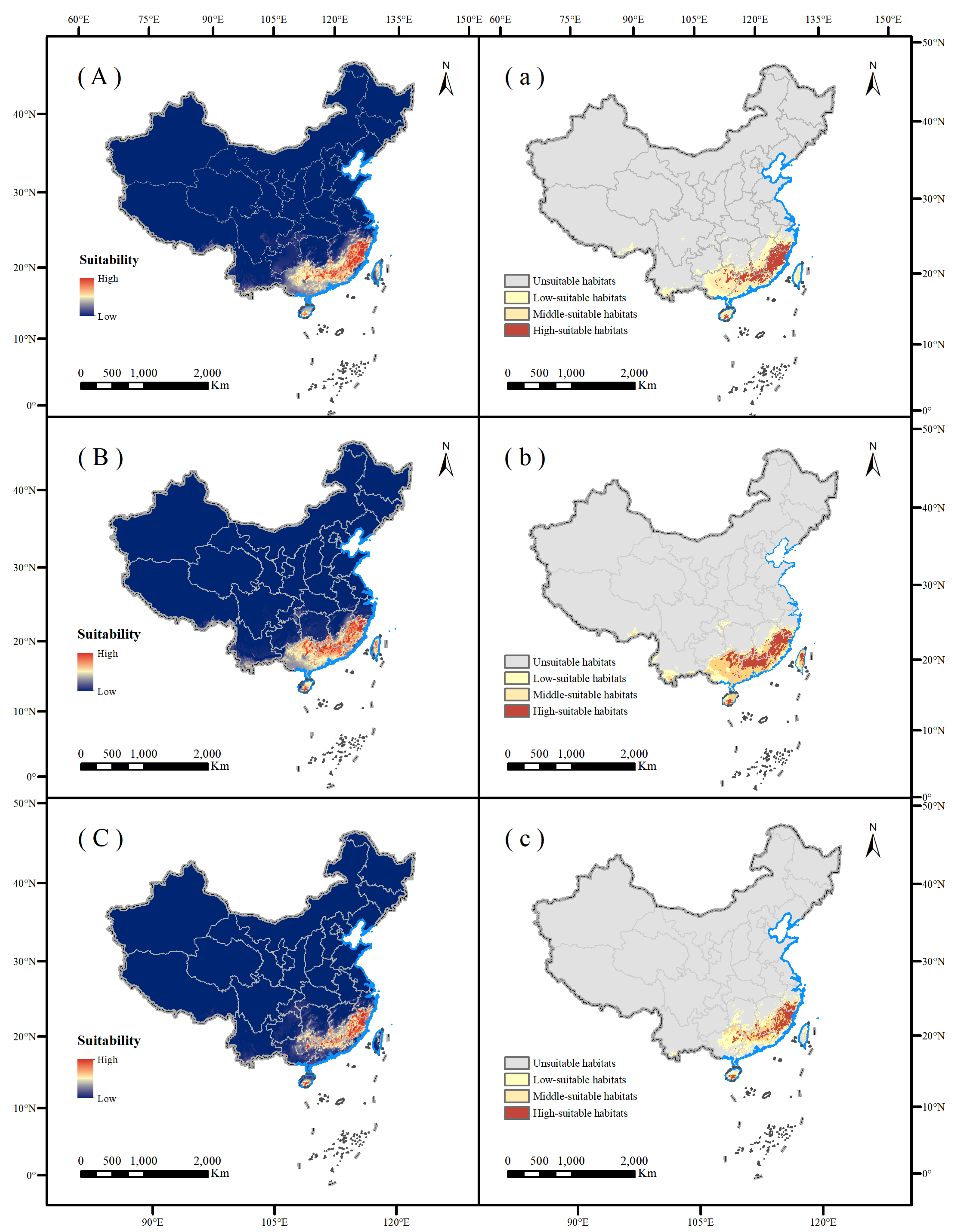
3.3. Prediction of the Suitable Zone of T. aureus
3.4. Migration Route of the Centroid in Future Suitable Areas
4. Discussion
4.1. Reliability of Habitat Suitability Prediction Results
4.2. Effects of Future Climate Scenarios on the Distribution of T. aureus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezeng, B.S.; Morales-Castilla, I.; van der Bank, M.; Yessoufou, K.; Daru, B.H.; Davies, T.J. Climate change may reduce the spread of non-native species. Ecosphere 2017, 8, 308–309. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 9 January 2024).
- Shen, X.X.; Cao, S.Y. The Sixth Assessment Report of the Intergovernmental Panel on Climate Change Calls for Urgent Climate Action to Ensure a Sustainable Future (International Perspective). Available online: http://world.people.com.cn/n1/2023/0404/c1002-32657063.html (accessed on 9 January 2024).
- Dong, Z.K.; Ge, F. The fitness of insects in response to climate warming. Chin. J. Appl. Entomol. 2011, 48, 1141–1148. [Google Scholar]
- Robert, P.A. A framework for using niche models to estimate impacts of climate change on species distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performanced epends onevaluation criteria: Dutch Hoverflie as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar]
- Tang, Q.H.; Zong, D.L.; Zhou, J.; Hu, X.; Wang, T. The potential threat and driving factors of cropland weeds Aegilops tauschii and Ambrosia artemisiifolia under global climate change. Chin. J. Ecol. 2022, 08, 1130. [Google Scholar]
- Liu, T.; Liu, Y.P.; Lu, T.; Liang, R.F.; Liu, F.; Ma, Z.; Zhou, Y.H.; Chen, Z.; Su, X. Potential distribution of Littledalea, an endemic genus from the Qinghai-Tibet plateau, predicted by Biomod2 models. Acta Agrestia Sin. 2020, 28, 1650–1656. [Google Scholar]
- Lin, B.Z.; Zhu, X.F.; Zeng, J.P.; Yuan, J.X. Research on biological characteristics of Teinoplpus aureus in Jiulianshan. For. Res. 2017, 30, 399–408. [Google Scholar]
- Cotton, A.; Racheli, T. A preliminary annotated checklist of the Papilionidae of Laos with notes on taxonomy, phenology, distribution and variation (Lepidoptera, Papilionoidea). Fragm. Entomol. 2006, 38, 279–378. [Google Scholar] [CrossRef]
- Xing, S.; Tsun, F.A.; Dufour, P.C.; Cheng, W.; Yuan, F.L.; Jia, F.; Lien, V.V.; Wang, M.; Bonebrakea, T.C. Conservation of data deficient species under multiple threats: Lessons from an iconic tropical butterfly (Teinopalpus aureus). Biol. Conserv. 2019, 234, 154–164. [Google Scholar] [CrossRef]
- Vu, V.L.; Le, Q.T.; Christoph, L.H. Diversity of swallowtal butterfly species (Ropalocra, Papilionidae) in three protected areas of Thua Thien Hue province. J. For. Sci. Technol. 2019, 07, 82–87. [Google Scholar]
- Zeng, J.; Zhou, S.; Ding, J.; Luo, B.T.; Qin, K. Behavior characteristics and habitat adaptabilities of the endangered butterfly Teinopalpus aureus in Mount Dayao. Acta Ecol. Sin. 2012, 32, 6527–6534. [Google Scholar] [CrossRef]
- Chen, R.L.; Cai, Y.S.; Gong, Y.N.; Gu, M.B. Two new hosts of Teinopalpus aureus Mell found in the Nanling National Nature Reserve. Guangdong For. Technol. 2009, 25, 119–120. [Google Scholar]
- Zeng, J.; Zhou, S.; Luo, B.; Qin, K.; Liang, Y.L. Morphology and bionomics of the endangered butterfly golden kaiserihind‚ Teinopalpus aureus‚ in Dayaoshan of Guangxi. Chin. Bull. Entomol. 2008, 45, 457–464. [Google Scholar]
- Zou, W.; Zeng, J.P.; Jiang, M.N.; Wang, L.; Zhou, S.Y.; Zhang, J.T. Problems of subspecies taxonomy of rare butterflies and their significance in conservation: With Teinopalpus Hope as examples. Acta Entomol. Sin. 2021, 64, 1338–1349. [Google Scholar]
- National Forestry and Grassland Administration; Ministry of Agriculture and Rural Affairs of the People’s Republic of China. (No. 3 of 2021) National List of Wildlife under Special Protection. Available online: http://www.gov.cn/xinwen/2021-02/09/content_5586227.htm (accessed on 9 January 2024).
- National Forestry and Grassland Administration. Outline of the 14th Five-Year Plan for Forestry and Grassland Protection and Development. Available online: https://www.forestry.gov.cn/c/www/lczc/44287.jhtml (accessed on 9 January 2024).
- Gimenez-Dixon, M. Teinopalpus aureus. The IUCN Red List of Threatened Species 1996. Available online: https://www.iucnredlist.org/ (accessed on 9 January 2024).
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of species–climate impact models under climate change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Xu, T.; Zong, S.X. Impact of Climate Change on the Habitat Suitability of Monochamus saltuarius Gebler (Coleoptera; Cerambycidae) and Its Natural Enemies in China. Forests 2024, 15, 33. [Google Scholar] [CrossRef]
- Zheng, H. The “Queen of Butterflies” Teinopalpus aureus Appeared in Taizhou for the First Time. Taizhou Daily, 16 May 2022. Available online: http://news.taizhou.com.cn/2022-05/16/content_6821334.htm (accessed on 9 January 2024).
- Huang, Y. Study on the Complete Mitochondrial Genomes of Teinopalpus aureus in Four Geographic Populations (Lepidoptera: Papilionidae) and Their Position of Phylogenetic Analysis; Guangxi Normal University: Guilin, China, 2014. [Google Scholar]
- Ye, Y.Z.; Yuan, Y.; Wang, H.X.; Ji, S.J.; Liu, L.H.; Wang, L.W. Description of 8 rare butterflies. China Sci. Technol. Inf. 2006, 20, 83–84+86. [Google Scholar]
- Xu, Q.; Jiang, F.; Lin, Y.; Huang, H.T.; Yao, L.Y. A brief account of the butterflies protected by state in Fujian. J. Fujian For. Sci. Technol. 2003, 30, 93–96. [Google Scholar]
- Zhang, H.F. Prediction of the potential sutable are of the rare and endangered species, Teinoplpus aureus in China. J. Jinggangshan Univ. (Nat. Sci.) 2023, 44, 56–62. [Google Scholar]
- Huang, Q.S. Teinopalpus aureus was discovered for the first time in Mangdangshan Nature Reserve. Fujian For. 2016, 12. [Google Scholar]
- Nie, X. The Distribution, Habitat Quality and Conservation Status of the Endangered Teinopalpus aureus in Jiangxi; Jiangxi Agricultural University: Nanchang, China, 2014. [Google Scholar]
- Zeng, J.; Lin, B.; Zhu, X.; Liu, L.Y. A host plant, Michelia maudiae, widespread–distributed in south China for the endangered butterfly of Teinopalpus aureus. Acta Agric. Univ. Jiangxiensis 2014, 36, 550–555. [Google Scholar]
- Ding, D.S.; Shi, M.; Zeng, Z.J.; Wang, A.Q.; Li, Z.H. Rare wild insects in Jiangxi (II). Jiang Xi Plant Prot. 2000, 23, 22–25. [Google Scholar]
- Chen, R.; Gu, M. The status and prospect of studies on Teinoplus aureus Mell. J. Environ. Entomol. 2009, 31, 80–84. [Google Scholar]
- Meng, L.; Mo, F.Y.; Tu, Z.; Zhou, S.Y.; Xue, Y.G. Discovery of a new distribution point of Teinopalpus aureus Mell in Guangxi, China. J. Guangxi Norm. Univ. (Nat. Sci. Ed.) 2016, 34, 134–136. [Google Scholar]
- He, D.; Jiang, G.; Yan, Z. Observations on copulatory and predatory behaviors of adult of Teinopalpus aureus Mell. Guangxi Sci. 2000, 7, 78–79. [Google Scholar]
- Mell, R. Noch unbeschriebene lepidopterenaus sudchina (II). Dtsch. Entomol. Z. 1923, 153–160. Available online: https://www.zobodat.at/pdf/Deutsche-Ent-Zeitschrift_1923_0153-0160.pdf (accessed on 9 January 2024).
- Zeng, F. Collection and preparation of butterfly specimens in Wuzhi mountain area. New Educ. 2012, 53. Available online: https://kns.cnki.net/kcms2/article/abstract?v=MuKJieOJLT0Cwsln0IEIyOwvErMlY1yJnXOYOXVR8VDUxvk927CVRhiTM0ERAU-8vGG75TWmUjO1HSVjB6_1pgd9xyg4dydWTJrV6NxADsHUlPrB5RKEDc3S6IVHcJ0rOj0mGCsWYVQ=&uniplatform=NZKPT&language=CHS (accessed on 9 January 2024).
- Jiang, T.; Jing, C.; Wang, Y.J.; Zhai, J.Q.; Cao, L.G.; Xu, X.W.; Yu, D.Y.; Su, B.D. Analysis of the possibility of achieving the Global Sustainable Development Goals under the SSPs path. Sci. Sin. Terrae 2020, 50, 1445–1454. [Google Scholar]
- Weng, Y.W.; Cai, W.J.; Wang, C. The application and future directions of the Shared Socioeconomic Pathways (SSPs). Clim. Change Res. 2020, 16, 215–222. [Google Scholar]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.G.; Li, L.; Li, W.P.; Jie, W.H.; Zhang, J.; Liu, Y.M.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to Maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 10, 1058–1069. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Ge, X.Z.; Zou, Y.; Guo, S.W.; Wang, T.; Tao, J.; Zong, S.X. Prediction of the potential geographical distribution of Hylurgus ligniperda at the global scale and in China using the Maxent model. J. Beijing For. Univ. 2022, 44, 90–99. [Google Scholar]
- Franklin, J. Mapping Species Distributions—Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Peterson, A.; Soberon, J.; Pearson, R.; Robert, P.A.; Martínez-Meyer, E.; Nakamura, M.; Miguel, B.A. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, P.R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Mateo, R.G.; Croat, T.B.; Felicísimo, Á.M.; Muñoz, J. Profile or group discriminative techniques? Generating reliable species distribution models using pseudo-absences and target-group absences from natural history collections. Divers. Distrib. 2010, 16, 84–94. [Google Scholar] [CrossRef]
- Mirza, Č.; Jasmijn, R.; Daniela, R.; Janse, J.H.; Huijbregts, M.A.J.; Schipper, A.M. On the importance of predictor choice, modelling technique, and number of pseudo-absences for bioclimatic envelope model performance. Ecol. Evol. 2020, 10, 12307–12317. [Google Scholar]
- Bi, Y.Q.; Zhang, M.X.; Chen, Y.; Wang, A.X.; Li, M.H. Applying Biomod2 for modeling of species suitable habitats: A case study of Paeonia lactiflora in China. China J. Chin. Mater. Medica 2022, 47, 376–384. [Google Scholar]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365–372. [Google Scholar]
- Pavlovi, L.; Stojanovic, D.; Mladenovi, E.; Lakicevic, M.; Orlovic, S. Potential elevation shift of the European beech stands (Fagus sylvatica L.) in Serbia. Front. Plant Sci. 2019, 10, 436609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N. Modelling the suitable habitat distribution of Magnolia officinalis using Ensemble Model. J. Sichuan Agric. Univ. 2019, 37, 481–489. [Google Scholar]
- Allouche, O.; Tsoa, R.A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2010, 43, 1223–1232. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Wang, Z.; Jing, H.L.; Fan, B.G. Prediction of potential distribution of Ziziphus jujuba var. spinosa in China under context of climate change. Sci. Silvae Sin. 2021, 57, 158–168. [Google Scholar]
- Rödder, D.; Schmitt, T.; Gros, P.; Ulrich, W.; Habel, J.C. Climate change drives mountain butterflies towards the summits. Sci. Rep. 2021, 11, 14382. [Google Scholar] [CrossRef]
- Habel, J.C.; Rödder, D.; Schmitt, T.; Neve, G. Global warming will affect the genetic diversity and uniqueness of Lycaena helle population. Glob. Change Biol. 2011, 17, 194–205. [Google Scholar] [CrossRef]
- Filazzola, A.; Matter, S.F.; Roland, J. Inclusion of trophic interactions increases the vulnerability of an alpine butterfy species to climate change. Glob. Change Biol. 2020, 26, 2867–2877. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Chen, C.Q.; Wang, J.Q.; Yang, J.; Liang, Y.; Song, Y.Z.; Liu, T.M. Apreliminary report on the investigation of Tenopalus aureus in Jinggangshan country, Jiangxi province. Anhui Agric. Sci. Bull. 2007, 13, 148–149. [Google Scholar]
- Wang, L. Multi-Scale Habitat Preference Research Based on Hostplant of the Butterfly Teinopalpus aureus; Jiangxi Agricultural University: Nanchang, China, 2023. [Google Scholar]
- Jiang, M. Larval Diet Selection of the Rare Butterfly of Teinopalpus aureus and Genetic Diversity of Two Magnoliaceae Hostplants; Jiangxi Agricultural University: Nanchang, China, 2022. [Google Scholar]







| Province | Location | Longitude (E) | Latitude (N) | Sources |
|---|---|---|---|---|
| Zhejiang | Wuyanling National Nature Reserve | 119.673889 | 27.719722 | [24] |
| Fushan Township, Huangyan District, Taizhou city | 120.887222 | 28.528056 | [24] | |
| Jingning She Nationality Autonomous County | 119.480833 | 27.878056 | [25] | |
| Chimu Mountain of Jingning country | 119.661667 | 27.924722 | [26] | |
| Fujian | Wuyi Mountain national nature reserve | 117.753889 | 27.650278 | [27] |
| Dayun Mountain National Nature Reserve | 118.175556 | 25.673333 | [27] | |
| Meihua Mountatin National Nature Reserve | 116.895795 | 25.294767 | [27] | |
| Wuping County, Longyan City | 116.695833 | 24.978056 | [27] | |
| Liancheng County, Longyan City | 116.878889 | 25.215833 | [27] | |
| Qitai Mountain Provincial Nature Reserve | 117.581944 | 26.948889 | [27] | |
| Jian ’ou city Jiyang town, Nanping City | 118.13741 | 27.13868 | [27] | |
| Yongchun County, Quanzhou City | 118.29424 | 25.32188 | [27] | |
| Teng Mountain Provincial Nature Reserve | 118.93258 | 25.86672 | [28] | |
| Xiyang Village, Giring Town, Yongtai County | 119.012778 | 25.772778 | [28] | |
| Tianbaoyan National Nature Reserve | 117.54574 | 25.93414 | [28] | |
| Niumu Provincial Nature Reserve | 117.468611 | 25.851389 | [27] | |
| Xianyang town, Quanzhou City, Yongchun County | 117.916111 | 25.409339 | [27] | |
| Mandang Mountain National Nature Reserve | 118.105833 | 26.680278 | [29] | |
| Shuiji town, Jianyang district, Nanping city | 118.179056 | 27.375248 | GBIF | |
| Jiangxi | Jinggang Mountain Provincial Nature Reserve | 114.15 | 26.55 | [15] |
| Wuyi Mountain National Nature Reserve | 117.716667 | 27.815278 | [30] | |
| Jiulian Mountain National Nature Reserve | 114.561389 | 24.608611 | [30] | |
| Longnan County, Ganzhou City | 115.429722 | 24.694444 | [31] | |
| Taiyuan She township, Leadshan County, Shangrao city | 117.483056 | 27.950278 | [32] | |
| Tianzhu mountain township, Yanshan County, Shangrao city | 117.731111 | 27.986389 | [32] | |
| Pingshan Scenic spot | 115.439722 | 25.704444 | [25] | |
| Sanqingshan scenic spot | 118.073056 | 28.905833 | [25] | |
| Hunan | Mang Mountain Provincial Nature Reserve | 112.839722 | 24.909167 | [33] |
| Guangdong | Nanling National Nature Reserve | 113.04563 | 24.894098 | [3] |
| Northern mountainous area of Lianping County, Heyuan City | 114.472778 | 24.501667 | [3] | |
| Provincial Nature Reserve Management Office of South China Tiger Protection in Northern Guangdong | 113.629722 | 24.896667 | [33] | |
| Longnan City, Ganzhou City | 114.625833 | 24.925278 | [33] | |
| Guangxi | Tiane town, Tiane County, Hechi City | 107.12 | 24.87 | GBIF |
| Chengzhong district, Liuzhou City | 109.422454 | 24.314681 | GBIF | |
| Liuzhou Rongshui Miao autonomous county | 109.264722 | 25.192222 | [34] | |
| Rongan County, Liuzhou City | 109.756667 | 25.486389 | [33] | |
| Dayaoshan Nature reserve | 110.315833 | 24.025556 | [35] | |
| Dayaoshan Nature reserve | 110.248056 | 24.168056 | [35] | |
| Daming Mountain National Nature Reserve | 108.402222 | 23.501944 | [36] | |
| Hainan | Wuzhi Mountion National Nature Reserve | 109.543889 | 19.004444 | [37] |
| Shuiman township, Wuzhishan city | 109.533313 | 18.785278 | GBIF | |
| Jianfengling Nature Reserve | 108.868611 | 18.724167 | [33] |
| Variable | Description | Unit |
|---|---|---|
| bio4 | Temperature seasonality | °C |
| bio6 | Min. temperature of coldest month | °C |
| bio8 | Mean temperature of wettest quarter | °C |
| bio12 | Annual precipitation | mm |
| bio14 | Precipitation of driest month | mm |
| bio18 | Precipitation of warmest quarter | mm |
| bio19 | Precipitation of coldest quarter | mm |
| elev | Elevation | m |
| Item | Algorithm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANN | CTA | FDA | GAM | GBM | GLM | XGBOOST | MARS | MAXENT | RF | SRE | ESM | |
| AUC | 0.981 | 0.902 | 0.862 | 0.862 | 0.983 | 0.975 | 0.922 | 0.977 | 0.968 | 0.963 | 0.735 | 0.990 |
| TSS | 0.845 | 0.805 | 0.805 | 0.725 | 0.855 | 0.905 | 0.740 | 0.835 | 0.750 | 0.815 | 0.470 | 0.972 |
| High-Suitable Habitats | Middle-Suitable Habitats | Low-Suitable Habitats | Total-Suitable Habitats | |
|---|---|---|---|---|
| current (1970–2000) | 34.43 | 41.47 | 63.05 | 138.95 |
| 2050s SSP1_2.6 | 15.82 | 38.64 | 52.06 | 106.52 |
| 2070s SSP1_2.6 | 19.54 | 26.11 | 38.88 | 84.53 |
| 2090s SSP1_2.6 | 16.83 | 25.60 | 34.49 | 76.93 |
| 2050s SSP5_8.5 | 19.02 | 34.60 | 32.88 | 86.50 |
| 2070s SSP5_8.5 | 21.24 | 34.50 | 25.96 | 81.70 |
| 2090s SSP5_8.5 | 12.17 | 21.20 | 27.66 | 61.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Zong, S. Prediction of the Potential Distribution of Teinopalpus aureus Mell, 1923 (Lepidoptera, Papilionidae) in China Using Habitat Suitability Models. Forests 2024, 15, 828. https://doi.org/10.3390/f15050828
Liu Y, Zhang X, Zong S. Prediction of the Potential Distribution of Teinopalpus aureus Mell, 1923 (Lepidoptera, Papilionidae) in China Using Habitat Suitability Models. Forests. 2024; 15(5):828. https://doi.org/10.3390/f15050828
Chicago/Turabian StyleLiu, Yinghan, Xuemei Zhang, and Shixiang Zong. 2024. "Prediction of the Potential Distribution of Teinopalpus aureus Mell, 1923 (Lepidoptera, Papilionidae) in China Using Habitat Suitability Models" Forests 15, no. 5: 828. https://doi.org/10.3390/f15050828





