Porcine Circovirus Type 3 (PCV3) in Poland: Prevalence in Wild Boar Population in Connection with African Swine Fever (ASF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analyzed Biological Material
2.2. Detection of ASFV, PCV3, PCV4
2.2.1. Sample Preparation
2.2.2. ASFV
2.2.3. PCV3 and PCV4
2.3. Statistical Analysis and Logistic Regression Models
2.4. Virus Isolation (PCV3)
2.5. PCR and Sequencing (PCV3)
2.6. Phylogenetic Analysis (PCV3)
3. Results
3.1. Detection of ASFV, PCV3, PCV4
3.2. Logistic Regression Models
3.3. Virus Isolation
3.4. Conventional PCR and Genome Sequencing (PCV3)
3.5. Phylogenetic Analysis (PCV3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Poland/Główny Urząd Statystyczny. Dostawy na Rynek Krajowy Oraz Spożycie Niektórych Artykułów Konsumpcyjnych na 1 Mieszkańca w 2021 Roku. Informacje Sygnalne, 2022, 1–3./Statistics Poland. Domestic Deliveries and Consumption of Selected Consumer Goods per Capita in 2021. Available online: https://stat.gov.pl/en/topics/prices-trade/trade/domestic-deliveries-and-consumption-of-selected-consumer-goods-per-capita-in-2021,9,12.html (accessed on 1 March 2024).
- Frant, M.P.; Gal-Cisoń, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Woźniakowski, G.; Szczotka-Bochniarz, A. African Swine Fever in Wild Boar (Poland 2020): Passive and Active Surveillance Analysis and Further Perspectives. Pathogens 2021, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes. Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Frant, M.; Gal, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Woźniakowski, G. African Swine Fever Virus (ASFV) in Poland in 2019—Wild Boars: Searching Pattern. Agriculture 2021, 11, 45. [Google Scholar] [CrossRef]
- Stadejek, T.; Woźniak, A.; Miłek, D.; Biernacka, K. First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transbound. Emerg. Dis. 2017, 64, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Miłek, D.; Stadejek, T. Wide Range of the Prevalence and Viral Loads of Porcine Circovirus Type 3 (PCV3) in Different Clinical Materials from 21 Polish Pig Farms. Pathogens 2020, 9, 411. [Google Scholar] [CrossRef]
- Segalés, J.; Sibila, M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Vet. Sci. 2022, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hou, L.; Zhou, J.; Wang, D.; Cui, Y.; Feng, X.; Liu, J. Porcine Circovirus Type 2 Vaccines: Commercial Application and Research Advances. Viruses 2022, 14, 2005. [Google Scholar] [CrossRef] [PubMed]
- Fabisiak, M.; Szczotka, A.; Podgórska, K.; Stadejek, T. Prevalence of infection and genetic diversity of porcine circovirus type 2 (PCV2) in wild boar (Sus scrofa) in Poland. J. Wildl. Dis. 2012, 48, 612–618. [Google Scholar] [CrossRef]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knustson, T.P.; Li, L.; Deng, X.; Resende, T.; Vanucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef]
- Sirisereewan, C.; Thanawongnuwech, R.; Kedkovid, R. Current Understanding of the Pathogenesis of Porcine Circovirus 3. Pathogens 2022, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mai, J.; Yang, Y.; Xiao, C.-T.; Wang, N. Current knowledge on epidemiology and evolution of novel porcine circovirus 4. Vet. Res. 2022, 53, 38. [Google Scholar] [CrossRef] [PubMed]
- Mora-Díaz, J.; Piñeyro, P.; Shen, H.; Schwartz, K.; Vannucci, F.; Li, G.; Arruda, B.; Giménez-Lirola, L. Isolation of PCV3 from Perinatal and Reproductive Cases of PCV3-Associated Disease and In Vivo Characterization of PCV3 Replication in CD/CD Growing Pigs. Viruses 2020, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Dias-Alves, A.; Cabezón, O.; Mentaberre, G.; Castillo-Contreras, R.; López-Béjar, M.; Casas-Díaz, E.; Sibila, M.; Correa-Fiz, F.; Segalés, J. Porcine circovirus 3 is highly prevalent in serum and tissues and may persistently infect wild boar (Sus scrofa scrofa). Transbound. Emerg. Dis. 2019, 66, 91–101. [Google Scholar] [CrossRef]
- Prinz, C.; Stillfried, M.; Neubert, L.K.; Denner, J. Detection of PCV3 in German wild boars. Virol. J. 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Pellegrini, F.; Camero, M.; Catella, C.; Buonavoglia, D.; Fusco, G.; Martella, V.; Lanave, G. Genetic Diversity of Porcine Circovirus Types 2 and 3 in Wild Boar in Italy. Animals 2022, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Ruiz, A.; Grassi, L.; Sibila, M.; Drigo, M.; Segalés, J. Lack of Porcine circovirus 4 Genome Detection in Pig Samples from Italy and Spain. Pathogens 2020, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Dundon, W.G.; Franzo, G.; Settypalli, T.B.K.; Dharmayanti, N.L.P.I.; Ankhanbaatar, U.; Sendow, I.; Ratnawati, A.; Sainnokhoi, T.; Molin, U.; Cattoli, G.; et al. Evidence of coinfection of pigs with African swine fever virus and porcine circovirus 2. Arch. Virol. 2022, 167, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Anahory, I.V.; Franzo, G.; Settypalli, T.B.K.; Mapaco, L.P.; Achá, S.J.; Molini, U.; Cattoli, G.; Lamien, C.E.; Dundon, W.G. Identification of porcine circovirus-3 in Mozambique. Vet. Res. Commun. 2022, 46, 593–596. [Google Scholar] [CrossRef]
- Luka, P.D.; Adedeji, A.J.; Jambol, A.R.; Ifende, I.V.; Luka, H.G.; Choji, N.D.; Weka, R.; Settypalli, T.B.K.; Achenbach, J.E.; Cattoli, G.; et al. Coinfections of African swine fever virus, porcine circovirus 2 and 3, and porcine parvovirus 1 in swine in Nigeria. Arch. Virol. 2022, 167, 2715–2722. [Google Scholar] [CrossRef]
- Krüger, L.; Längin, M.; Reichart, B.; Fiebig, U.; Kristiansen, Y.; Prinz, C.; Kessler, B.; Egerer, S.; Wolf, E.; Abicht, J.M.; et al. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses 2019, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for Growing and Titrating African Swine Fever Virus: Field and Laboratory Samples. Curr. Protoc. Cell Biol. 2011, 53, 26.14.1–26.14.25. [Google Scholar] [CrossRef] [PubMed]
- Fux, R.; Söckler, C.; Link, E.K.; Renken, C.; Krejci, R.; Sutter, G.; Ritzman, M.; Eddicks, M. Full genome characterization of porcine circovirus type 3 isolates reveals the existence of two distinct groups of virus strains. Virol. J. 2018, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Bocian, Ł.; Frant, M.; Ziętek-Barszcz, A.; Niemczuk, K.; Szczotka-Bochniarz, A. Dynamics of the African Swine Fever Spread in Poland. J. Vet. Res. 2022, 66, 459–471. [Google Scholar] [CrossRef]
- Szymańska, E.J.; Dziwulaki, M. Development of African Swine Fever in Poland. Agriculture 2022, 12, 119. [Google Scholar] [CrossRef]
- Pejsak, Z.; Tarasiuk, K. Eight years of African swine fever in Poland. Med. Weter. 2022, 78, 481–488. [Google Scholar] [CrossRef]
- Frant, M.P.; Gal-Cisoń, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Szczotka-Bochniarz, A. African Swine Fever (ASF) Trend Analysis in Wild Boar in Poland (2014–2020). Animals 2022, 12, 1170. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.M.M.G.; Xiao, C.-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef]
- Arruda, B.; Piñeyro, P.; Derscheid, R.; Hause, B.; Byers, E.; Dion, K.; Long, D.; Sievers, C.; Tangen, J.; Williams, T.; et al. PCV3-associated disease in the United States swine herd. Emerg. Microbes. Infect. 2019, 8, 684–698. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine Circovirus Type 2 and Porcine Circovirus-Associated Disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Han, Z.; Liu, S. Epidemiological investigation of porcine circovirus type 2 and its coinfection rate in Shandong province in China from 2015 to 2018. BMC Vet. Res. 2021, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Pallares, F.J.; Halbur, P.G.; Opriessnig, T.; Sorden, S.D.; Villar, D.; Janke, B.H.; Yaeger, M.J.; Larson, D.J.; Schwartz, K.J.; Yoon, K.J.; et al. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Investig. 2002, 14, 515–519. [Google Scholar] [CrossRef]
- Guo, Z.; Ruan, H.; Qiao, S.; Deng, R.; Zhang, G. Co-infection status of porcine circoviruses (PCV2 and PCV3) and porcine epidemic diarrhea virus (PEDV) in pigs with watery diarrhea in Henan province, central China. Microb. Pathog. 2020, 142, 104047. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, L.; Hu, X.; Zhao, S.; Xu, P.; Li, W.; Chen, J.; Zhang, Y.; Xia, P. Immune Molecules’ mRNA Expression in Porcine Alveolar Macrophages Co-Infected with Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2. Viruses 2023, 15, 777. [Google Scholar] [CrossRef]
- Oh, T.; Chae, C. First isolation and genetic characterization of porcine circovirus type 3 using primary porcine kidney cells. Vet. Microbiol. 2020, 241, 108576. [Google Scholar] [CrossRef] [PubMed]
- Klaumann, F.; Correa-Fiz, F.; Franzo, G.; Sibila, M.; Núñez, J.I.; Segalés, J. Current Knowledge on Porcine circovirus 3 (PCV-3): A Novel Virus with a Yet Unknown Impact on the Swine Industry. Front. Vet. Sci. 2018, 5, 315. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Delwart, E.; Fux, R.; Hause, B.; Su, S.; Zhou, J.; Segalés, J. Genotyping Porcine Circovirus 3 (PCV-3) Nowadays: Does It Make Sense? Viruses 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
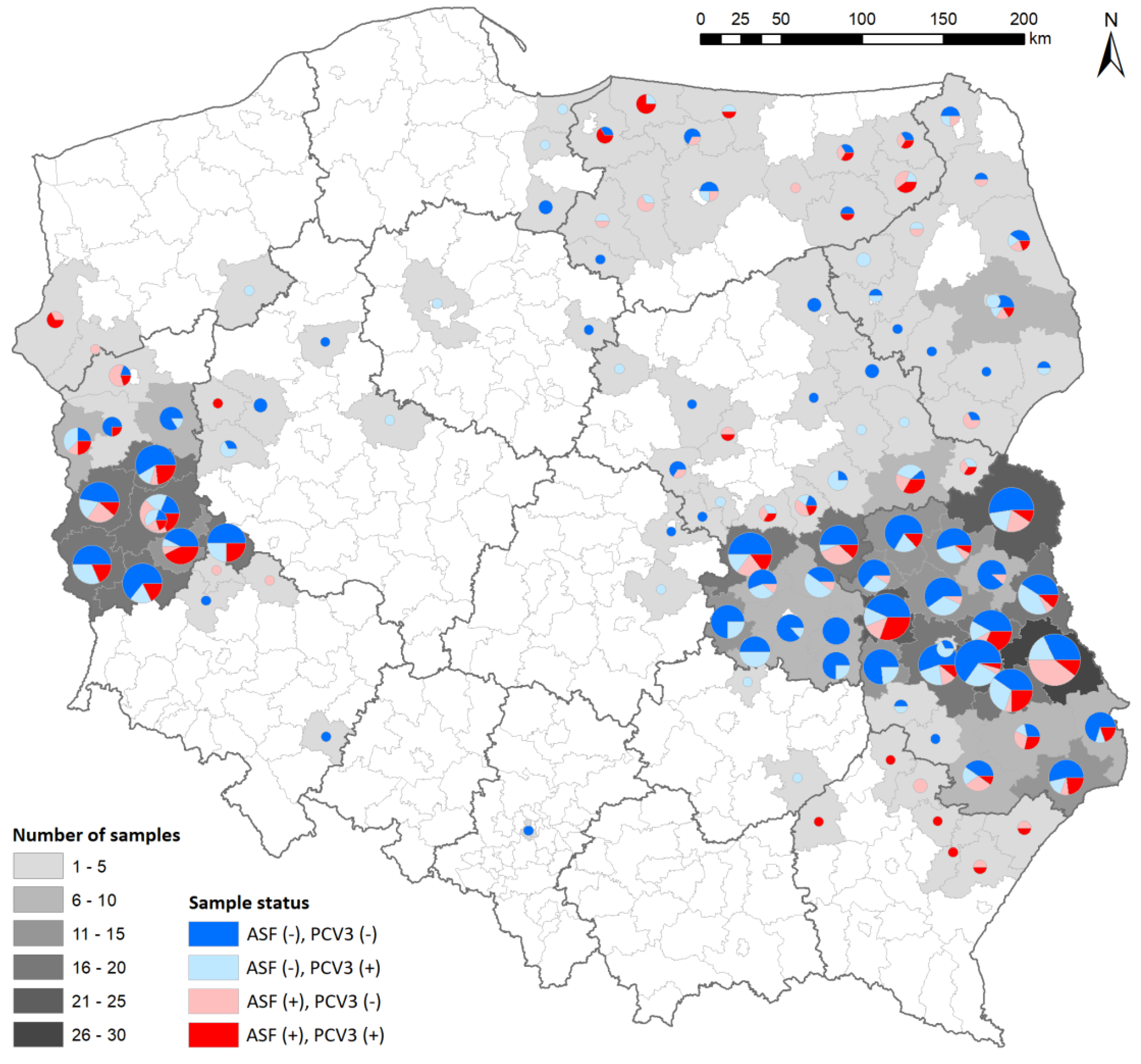
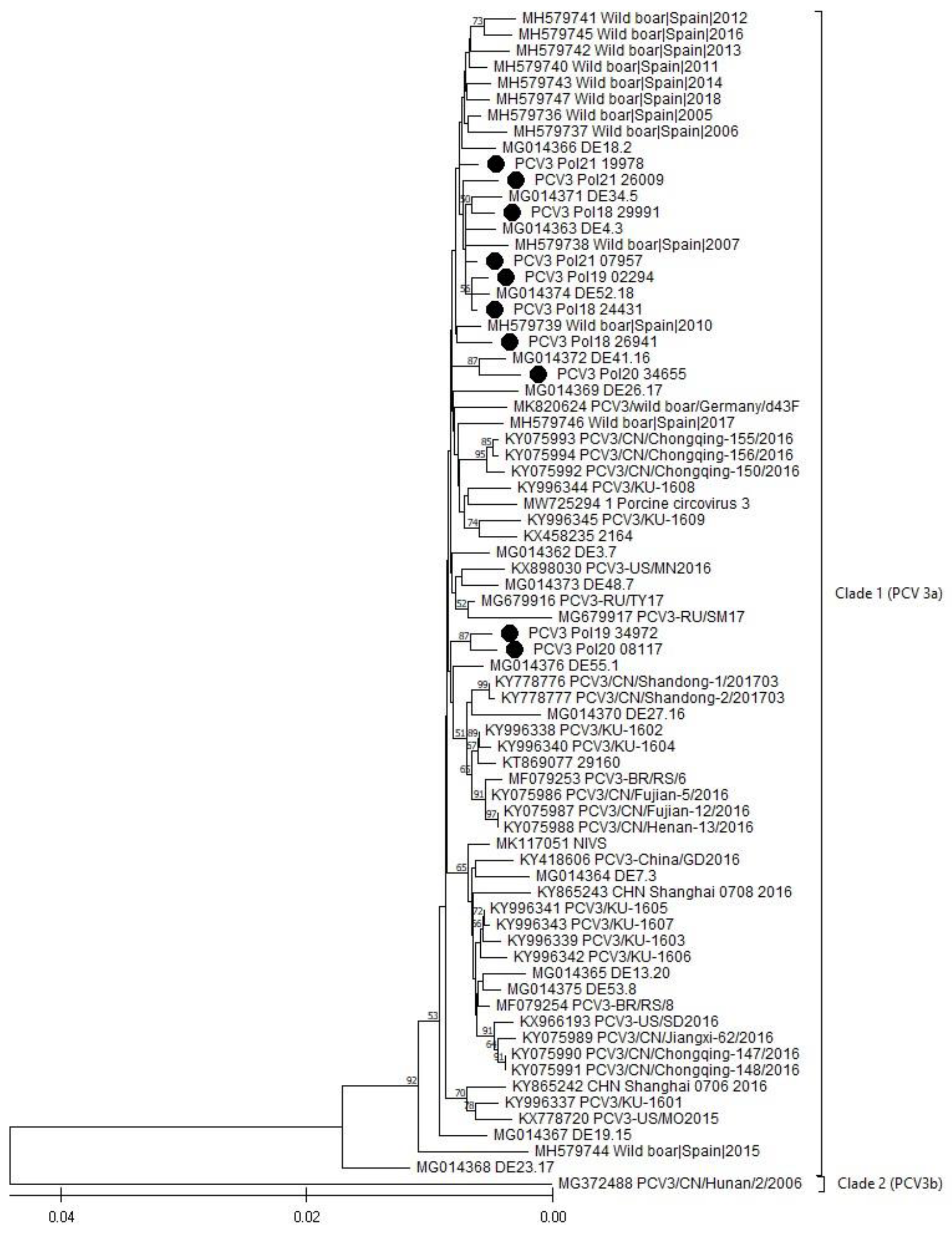
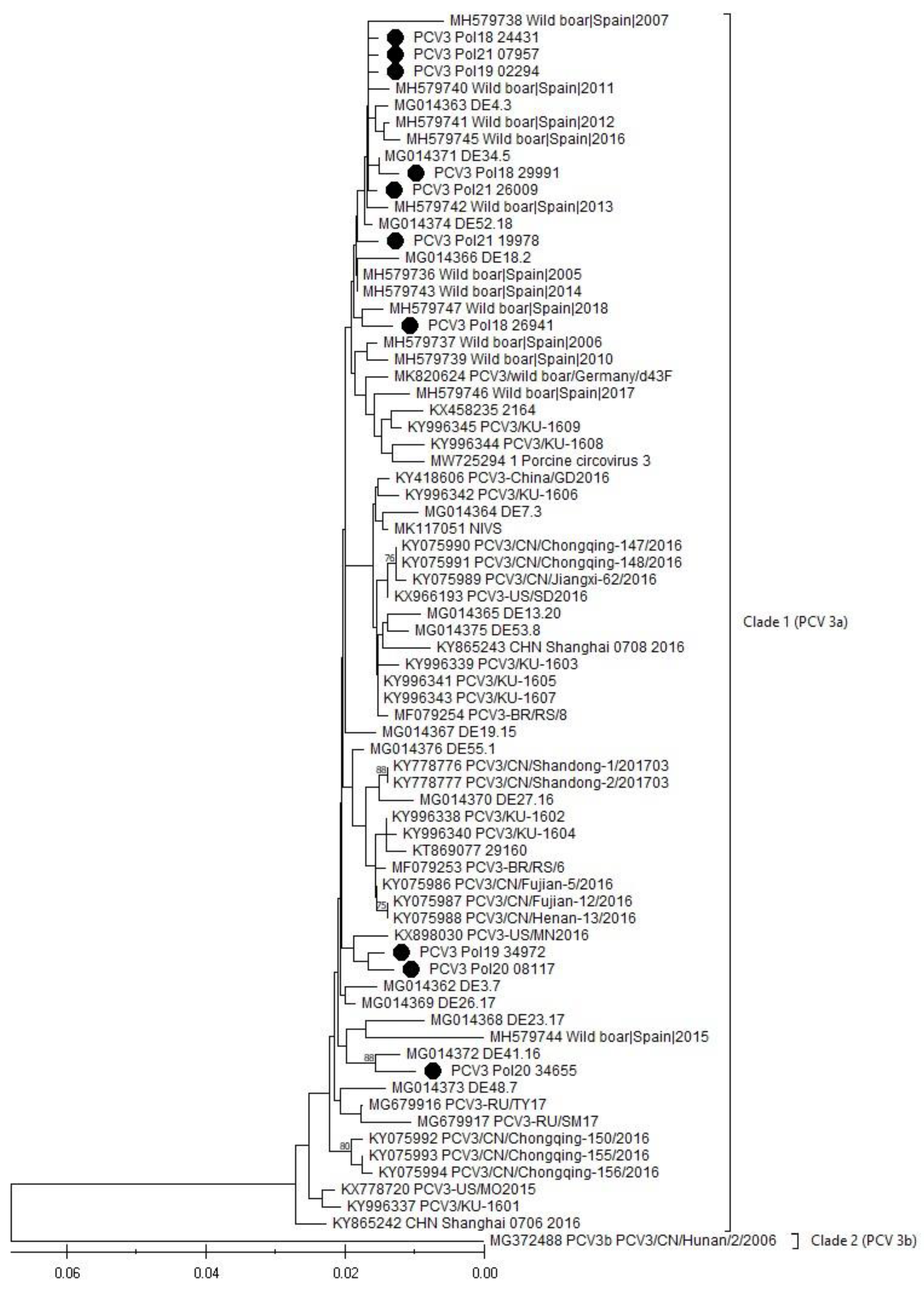
| Voivodship | Group I (ASFV−) | Group II (ASFV+) | Total |
|---|---|---|---|
| Dolnośląskie | 2 | 2 | 4 |
| Kujawsko-Pomorskie | 2 | 0 | 2 |
| Lubelskie | 229 | 73 | 302 |
| Lubuskie | 82 | 49 | 131 |
| Łódzkie | 1 | 0 | 1 |
| Mazowieckie | 112 | 30 | 142 |
| Podkarpackie | 0 | 10 | 10 |
| Podlaskie | 24 | 9 | 33 |
| Pomorskie | 3 | 0 | 3 |
| Śląskie | 1 | 0 | 1 |
| Świętokrzyskie | 2 | 0 | 2 |
| Warmińsko-Mazurskie | 14 | 23 | 37 |
| Wielkopolskie | 7 | 0 | 7 |
| Zachodniopomorskie | 1 | 4 | 5 |
| Total | 480 | 200 | 680 |
| PCR Target | Name | Type | Sequence (5′-3′) | Nucleotide Position ** | Length [bp] |
|---|---|---|---|---|---|
| PCV3 Cap | PCV3-Cap-F * | primer | AGTGCTCCCCATTGAACG | 1434–1451 | 18 |
| PCV3-Cap-R * | primer | ACACAGCCGTTACTTCAC | 1551–1568 | 18 | |
| PCV3-probe * | probe | 6FAM-ACCCCATGGCTCAACACATATGACC-BHQ1 | 1456–1480 | 25 | |
| PCV3-control | synthetic DNA | AGT GCT CCC CAT TGA ACG GTG GGG TCA TAT GTG TTG AGC CAT GGG GTG GGT CTG GAG GAG AAA AAG AGG CTT TGT CCT GGG TGA GCG CTG GTA GTT CCC GCC AGA ATT GGT TTG GGG GTG AAG TAA CGG CTG TGT | 1434–1568 | 135 | |
| PCV4 Cap | PCV4-Cap-F | primer | AGGTTTACGATCGTTCCC | 1486–1503 | 18 |
| PCV4-Cap-R | primer | CTTCATGAGGGAGGTGAC | 1588–1605 | 18 | |
| PCV4-probe | probe | 6FAM-TAATGTCCAACGTTCCAAGAGGGCG-BHQ1 | 1540–1564 | 25 | |
| PCV4-control | synthetic DNA | CTG TAA AGG TTT ACG ATC GTT CCC GGT CCT TTT GGG ATA AAG TCC TTC AGT TTG AAA TCG TAA TGT CCA ACG TTC CAA GAG GGC GTG GAA AAG CTT GAC ACG CTG AGA GTC ACC TCC CTC ATG AAG CGC GCAT | 1492–1624 | 133 |
| Primer | Sequence (5′-3′) | Length of Amplified Sequence [bp] | Annealing Temperature [°C] | Extension Time [s] |
|---|---|---|---|---|
| PCV374 F * | CACCGTGTCAGTGGATATAC | 1072 | 55 | 120 |
| PCV31144 R * | CACCCCAACGCAATAATTGTA | |||
| PCV31137 F * | TTGGGGTGGGGGTATTTATT | 425 | 55 | 120 |
| PCV31561 R * | ACACAGCCGTTACTTCAC | |||
| PCV31427 F * | AGTGCTCCCCATTGAACG | 1007 | 55 | 120 |
| PCV3433 R * | CGACCAAATCCGGGTAAGC | |||
| PCV3911 F | GGGTTCCTGTTAAGGGTGGG | 487 | 60 | 30 |
| PCV31415 R | GACAGACTTCTACGGCACCA |
| Year/Voivodship | Group I-ASFV (−) | Group II-ASFV (+) | Total | ||||
|---|---|---|---|---|---|---|---|
| PCV3 (−) | PCV3 (+) | Total | PCV3 (−) | PCV3 (+) | Total | ||
| 2018 | 35 | 45 | 80 | 14 | 8 | 22 | 102 |
| 2019 | 115 | 45 | 160 | 27 | 21 | 48 | 208 |
| 2020 | 120 | 40 | 160 | 21 | 29 | 50 | 210 |
| 2021 | 56 | 24 | 80 | 35 | 45 | 80 | 160 |
| Dolnośląskie | 2 | 0 | 2 | 2 | 0 | 2 | 4 |
| Kujawsko-Pomorskie | 1 | 1 | 2 | 0 | 0 | 0 | 2 |
| Lubelskie | 161 | 68 | 229 | 36 | 37 | 73 | 302 |
| Lubuskie | 58 | 24 | 82 | 17 | 32 | 49 | 131 |
| Łódzkie | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Mazowieckie | 74 | 38 | 112 | 18 | 12 | 30 | 142 |
| Podkarpackie | 0 | 0 | 0 | 4 | 6 | 10 | 10 |
| Podlaskie | 14 | 10 | 24 | 7 | 2 | 9 | 33 |
| Pomorskie | 2 | 1 | 3 | 0 | 0 | 0 | 3 |
| Śląskie | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Świętokrzyskie | 0 | 2 | 2 | 0 | 0 | 0 | 2 |
| Warmińsko-Mazurskie | 8 | 6 | 14 | 11 | 12 | 23 | 37 |
| Wielkopolskie | 4 | 3 | 7 | 0 | 0 | 0 | 7 |
| Zachodniopomorskie | 0 | 1 | 1 | 2 | 2 | 4 | 5 |
| Total | 326 | 154 | 480 | 97 | 103 | 200 | 680 |
| Name | Group | Year | Origin | Sample Type | Sample Ct (PCV3) | Voivodship | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| PCV3_Pol18_24431 | I | 2018 | road killed | lung | 24.11 | Mazowieckie | OQ533868 |
| PCV3_Pol18_26941 | I | 2018 | found dead | spleen | 23.87 | Podlaskie | OQ533864 |
| PCV3_Pol18_29991 | I | 2018 | found dead | tonsil | 24.72 | Lubelskie | OQ533871 |
| PCV3_Pol19_02294 | II | 2019 | found dead | lung | 24.86 | Lubelskie | OQ533869 |
| PCV3_Pol19_34972 | II | 2019 | found dead | lung | 23.99 | Lubelskie | OQ533865 |
| PCV3_Pol20_08117 | II | 2020 | road killed | spleen | 23.86 | Lubuskie | OQ533866 |
| PCV3_Pol20_34655 | II | 2020 | found dead | lung | 25.17 | Lubuskie | OQ533872 |
| PCV3_Pol21_07957 | II | 2021 | found dead | spleen | 26.33 | Podkarpackie | OQ533870 |
| PCV3_Pol21_19978 | II | 2021 | found dead | spleen | 25.27 | Lubuskie | OQ533873 |
| PCV3_Pol21_26009 | II | 2021 | hunted | blood | 25.70 | Lubelskie | OQ533867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frant, M.P.; Mazur-Panasiuk, N.; Gal-Cisoń, A.; Bocian, Ł.; Łyjak, M.; Szczotka-Bochniarz, A. Porcine Circovirus Type 3 (PCV3) in Poland: Prevalence in Wild Boar Population in Connection with African Swine Fever (ASF). Viruses 2024, 16, 754. https://doi.org/10.3390/v16050754
Frant MP, Mazur-Panasiuk N, Gal-Cisoń A, Bocian Ł, Łyjak M, Szczotka-Bochniarz A. Porcine Circovirus Type 3 (PCV3) in Poland: Prevalence in Wild Boar Population in Connection with African Swine Fever (ASF). Viruses. 2024; 16(5):754. https://doi.org/10.3390/v16050754
Chicago/Turabian StyleFrant, Maciej Piotr, Natalia Mazur-Panasiuk, Anna Gal-Cisoń, Łukasz Bocian, Magdalena Łyjak, and Anna Szczotka-Bochniarz. 2024. "Porcine Circovirus Type 3 (PCV3) in Poland: Prevalence in Wild Boar Population in Connection with African Swine Fever (ASF)" Viruses 16, no. 5: 754. https://doi.org/10.3390/v16050754





