Trends in Targeted Therapy Usage in Inflammatory Bowel Disease: TRENDY Study of ENEIDA
Abstract
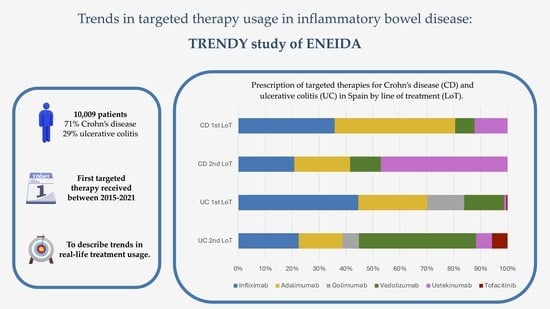
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Definitions
2.3.1. Targeted Therapies and Immunomodulatory Treatments
2.3.2. Change of Line
2.3.3. IASIST Score
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Crohn’s Disease
3.2.1. Patterns of Targeted Therapies by Line of Treatment
3.2.2. Patterns of Therapy Changes in Different Lines of Treatment
3.3. Ulcerative Colitis
3.3.1. Patterns of Targeted Therapies by Line of Treatment
3.3.2. Patterns of Therapy Changes in Different Lines of Treatment
3.4. Trends in Use of Biosimilars
3.5. Factors Influencing Treatment Choice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Cunningham, G.; Tamilarasan, A.G.; Irving, P.M. Positioning Biologics and New Therapies in the Management of Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2019, 35, 296–301. [Google Scholar] [CrossRef]
- Burisch, J.; Zhao, M.; Odes, S.; De Cruz, P.; Vermeire, S.; Bernstein, C.N.; Kaplan, G.G.; Duricova, D.; Greenberg, D.; Melberg, H.O.; et al. The Cost of Inflammatory Bowel Disease in High-Income Settings: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2023, 8, 458–492. [Google Scholar] [CrossRef]
- Zhao, M.; Sall Jensen, M.; Knudsen, T.; Kelsen, J.; Coskun, M.; Kjellberg, J.; Burisch, J. Trends in the Use of Biologicals and Their Treatment Outcomes among Patients with Inflammatory Bowel Diseases—A Danish Nationwide Cohort Study. Aliment. Pharmacol. Ther. 2022, 55, 541–557. [Google Scholar] [CrossRef]
- Sedano, R.; Almradi, A.; Ma, C.; Jairath, V.; Feagan, B.G. Novel Therapeutics for the Treatment of IBD: Current Status and Future Directions. Curr. Treat. Options Gastroenterol. 2020, 18, 442–461. [Google Scholar] [CrossRef]
- Chang, S.; Hudesman, D. First-Line Biologics or Small Molecules in Inflammatory Bowel Disease: A Practical Guide for the Clinician. Curr. Gastroenterol. Rep. 2020, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S.; Colombel, J.-F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. Ustekinumab versus Adalimumab for Induction and Maintenance Therapy in Biologic-Naive Patients with Moderately to Severely Active Crohn’s Disease: A Multicentre, Randomised, Double-Blind, Parallel-Group, Phase 3b Trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F.; Siegmund, B. Personalizing Treatment in IBD: Hype or Reality in 2020? Can We Predict Response to Anti-TNF? Front. Med. 2020, 7, 517. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Primary Failure to an Anti-Tnf Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-Tnf Agent) or Swap (for Another Mechanism of Action)? J. Clin. Med. 2021, 10, 5318. [Google Scholar] [CrossRef]
- Rogers, K.V.; Martin, S.W.; Bhattacharya, I.; Singh, R.S.P.; Nayak, S. A Dynamic Quantitative Systems Pharmacology Model of Inflammatory Bowel Disease: Part 2—Application to Current Therapies in Crohn’s Disease. Clin. Transl. Sci. 2021, 14, 249–259. [Google Scholar] [CrossRef]
- Lamb, C.A.; Saifuddin, A.; Powell, N.; Rieder, F. The Future of Precision Medicine to Predict Outcomes and Control Tissue Remodeling in Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1525–1542. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in Oral Nano-Delivery Systems for Colon Targeted Drug Delivery in Inflammatory Bowel Disease: Selective Targeting to Diseased versus Healthy Tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar]
- Zabana, Y.; Panés, J.; Nos, P.; Gomollón, F.; Esteve, M.; García-Sánchez, V.; Gisbert, J.P.; Barreiro-de-Acosta, M.; Domènech, E. The ENEIDA Registry (Nationwide Study on Genetic and Environmental Determinants of Inflammatory Bowel Disease) by GETECCU: Design, Monitoring and Functions. Gastroenterol. Hepatol. 2020, 43, 551–558. [Google Scholar] [PubMed]
- Ministerio de Sanidad, Gobierno de España. Catálogo Nacional de Hospitales 2022; Ministerio de Sanidad, Gobierno de España: Madrid, Spain, 2022. [Google Scholar]
- Brady, J.E.; Stott-Miller, M.; Mu, G.; Perera, S. Treatment Patterns and Sequencing in Patients with Inflammatory Bowel Disease. Clin. Ther. 2018, 40, 1509–1521.e5. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Han, M.; Park, S.; Cheon, J.H. Biologic Use Patterns and Predictors for Non-Persistence and Switching of Biologics in Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study. Dig. Dis. Sci. 2020, 65, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.J.; Jensen, C.B.; Wennerström, C.; Burisch, J.; Petersen, J. Drug Utilization of Biologic Therapy in Crohn’s Disease and Ulcerative Colitis: A Population-Based Danish Cohort Study 2015–2020. Scand. J. Gastroenterol. 2023, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; DeWoody, K.L.; et al. Infliximab for the Treatment of Fistulas in Patients with Crohn’s Disease. N. Engl. J. Med. 1999, 340, 1398–1405. [Google Scholar] [CrossRef]
- Singh, S.; Proctor, D.; Scott, F.I.; Falck-Ytter, Y.; Feuerstein, J.D. AGA Technical Review on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2512–2556.e9. [Google Scholar] [CrossRef]
- Laredo, V.; Gargallo-Puyuelo, C.J.; Gomollón, F. How to Choose the Biologic Therapy in a Bio-Naïve Patient with Inflammatory Bowel Disease. J. Clin. Med. 2022, 11, 829. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Van Assche, G.; Reinisch, W.; Colombel, J.; D’Haens, G.; Wolf, D.C.; Kron, M.; Tighe, M.B.; Lazar, A.; Thakkar, R.B. Adalimumab Induces and Maintains Clinical Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2012, 142, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Murad, M.H.; Fumery, M.; Dulai, P.S.; Sandborn, W.J. First- and Second-Line Pharmacotherapies for Patients With Moderate to Severely Active Ulcerative Colitis: An Updated Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 2179–2191.e6. [Google Scholar] [CrossRef] [PubMed]
- Järnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlén, P.; Grännö, C.; Vilien, M.; Ström, M.; Danielsson, Å.; Verbaan, H.; et al. Infliximab as Rescue Therapy in Severe to Moderately Severe Ulcerative Colitis: A Randomized, Placebo-Controlled Study. Gastroenterology 2005, 128, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of Vedolizumab Induction Therapy for Patients with Crohn’s Disease in Whom Tumor Necrosis Factor Antagonist Treatment Failed. Gastroenterology 2014, 147, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Townsend, T.; Razanskaite, V.; Dodd, S.; Storey, D.; Michail, S.; Morgan, J.; Davies, M.; Penman, D.; Watters, C.; Swaminathan, M.; et al. Comparative Effectiveness of Ustekinumab or Vedolizumab after One Year in 130 Patients with Anti-TNF-Refractory Crohn’s Disease. Aliment. Pharmacol. Ther. 2020, 52, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Biemans, V.B.C.; van der Woude, C.J.; Dijkstra, G.; van der Meulen-de Jong, A.E.; Löwenberg, M.; de Boer, N.K.; Oldenburg, B.; Srivastava, N.; Jansen, J.M.; Bodelier, A.G.L.; et al. Ustekinumab Is Associated with Superior Effectiveness Outcomes Compared to Vedolizumab in Crohn’s Disease Patients with Prior Failure to Anti-TNF Treatment. Aliment. Pharmacol. Ther. 2020, 52, 123–134. [Google Scholar] [CrossRef]
- Manlay, L.; Boschetti, G.; Pereira, B.; Flourié, B.; Dapoigny, M.; Reymond, M.; Sollelis, E.; Gay, C.; Boube, M.; Buisson, A.; et al. Comparison of Short- and Long-Term Effectiveness between Ustekinumab and Vedolizumab in Patients with Crohn’s Disease Refractory to Anti-Tumour Necrosis Factor Therapy. Aliment. Pharmacol. Ther. 2021, 53, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.D.; Ainsworth, C.; Mody, R.; Bergman, A.; Ling, C.S.; Medjedovic, J.; Smyth, M. Systematic Review with Network Meta-Analysis: Comparative Efficacy of Biologics in the Treatment of Moderately to Severely Active Ulcerative Colitis. PLoS ONE 2016, 11, e0165435. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Mínguez, M.; Ricart, E.; Taxonera, C.; García-López, S.; Guardiola, J.; López-San Román, A.; Iglesias, E.; Beltrán, B.; et al. Effectiveness and Safety of the Sequential Use of a Second and Third Anti-TNF Agent in Patients with Inflammatory Bowel Disease: Results from the Eneida Registry. Inflamm. Bowel Dis. 2020, 26, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Solitano, V.; D’Amico, F.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Biosimilar Switching in Inflammatory Bowel Disease: From Evidence to Clinical Practice. Expert Rev. Clin. Immunol. 2020, 16, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Nantes, Ó.; Varela, P.; Vela-González, M.; Rivero, M.; Sierra-Gabarda, O.; Riestra, S.; Acosta, M.B.-d.; Martín-Rodríguez, M.d.M.; Gargallo-Puyuelo, C.J.; et al. Real-world Outcomes of Switching from Adalimumab Originator to Adalimumab Biosimilar in Patients with Inflammatory Bowel Disease: The ADA-SWITCH Study. Aliment. Pharmacol. Ther. 2023, 58, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, H.; Yasmin, F.; Surani, S. Emerging Role of Biosimilars in the Clinical Care of Inflammatory Bowel Disease Patients. World J. Clin. Cases 2022, 10, 4327–4333. [Google Scholar] [CrossRef] [PubMed]




| Crohn’s Disease (n = 7089) | Ulcerative Colitis (n = 2920) | |
|---|---|---|
| Male gender, n (%) | 3657 (52) | 1580 (54) |
| Mean age at diagnosis (SD) | 43 (16) | 45 (16) |
| Extraintestinal manifestations, n (%) | 1958 (29) | 621 (22) |
| Smoking history, n (%) Smoker at diagnosis Ex-smoker | 3393 (52) 2844 (44) 549 (8) | 917 (35) 418 (16) 499 (19) |
| Family history, n (%) | 1120 (17) | 364 (14) |
| Surgery treatment a, n (%) Abdominal Perianal Both | 3120 (44) 2076 (66) 697 (22) 384 (12) | 392 (13) |
| Montreal location at Crohn’s disease diagnosis, n (%) | ||
| L1 (ileal) L2 (colonic) L3 (ileocolonic) L4 (upper gastrointestinal tract) | 1899 (29) 936 (14) 2918 (45) 737 (11) | |
| Montreal behavior at Crohn’s disease diagnosis, n (%) | ||
| B1 (inflammatory) B2 (stricturing) B3 (fistulising) | 3933 (55) 1656 (23) 1500 (21) | |
| Perianal disease, n (%) | 2128 (30) | |
| Ulcerative colitis extension, n (%) | ||
| E1 (proctitis) E2 (left-sided colitis) E3 (extensive colitis) | 189 (6) 1136 (39) 1576 (54) | |
| Prior or concomitant use of immunomodulators, n (%) | 5765 (81) | 2254 (77) |
| Lines of treatment with targeted therapies, n (%) | ||
| 1st LoT 2nd LoT 3rd LoT or more b | 5014 (71) 1533 (22) 542 (7) | 1820 (62) 681 (23) 418 (15) |
| A. Crohn’s Disease | 1st LoT | 2nd LoT | 3rd LoT | 4th LoT | 5th LoT | 6th LoT | Total |
|---|---|---|---|---|---|---|---|
| anti-TNFα, n (%) | 5698 (80.5) | 864 (41.5) | 141 (26.1) | 34 (28.1) | 10 (38.5) | 0 (0.0) | 6747 (68.5) |
| Infliximab, n (%) | 2527 (35.7) | 431 (20.7) | 81 (14.9) | 24 (19.9) | 6 (23.1) | 0 (0.0) | 3069 (31.2) |
| Infliximab originator | 508 (7.2) | 65 (3.1) | 13 (2.4) | 2 (1.7) | 0 (0.0) | 0 (0.0) | 588 (6.0) |
| Infliximab biosimilar | 2019 (28.5) | 366 (17.6) | 68 (12.5) | 22 (18.2) | 6 (23.1) | 0 (0.0) | 2481 (25.2) |
| Adalimumab, n (%) | 3158 (44.6) | 428 (20.6) | 57 (10.6) | 10 (8.2) | 4 (15.4) | 0 (0.0) | 3657 (37.1) |
| Adalimumab originator | 2026 (28.6) | 293 (14.1) | 28 (5.2) | 5 (4.1) | 2 (7.7) | 0 (0.0) | 2354 (23.9) |
| Adalimumab biosimilar | 1132 (16.0) | 135 (6.5) | 29 (5.4) | 5 (4.1) | 2 (7.7) | 0 (0.0) | 1303 (13.2) |
| Vedolizumab, n (%) | 520 (7.3) | 236 (11.4) | 110 (20.3) | 33 (27.3) | 7 (26.9) | 1 (25.0) | 907 (9.2) |
| Ustekinumab, n (%) | 868 (12.2) | 972 (46.8) | 287 (53.0) | 54 (44.6) | 8 (30.8) | 3 (75.0) | 2192 (22.2) |
| Overall, n | 7089 | 2075 | 542 | 121 | 26 | 4 | 9857 |
| B. Ulcerative Colitis | 1st LoT | 2nd LoT | 3rd LoT | 4th LoT | 5th LoT | 6th LoT | Total |
| anti-TNFα, n (%) | 2453 (83.9) | 492 (44.8) | 77 (18.4) | 18 (13.9) | 7 (17.5) | 1 (11.1) | 3048 (66.1) |
| Infliximab, n (%) | 1304 (44.6) | 246 (22.4) | 41 (9.8) | 12 (9.3) | 4 (10.0) | 0 (0.0) | 1607 (34.8) |
| Infliximab originator | 264 (9.0) | 49 (4.5) | 10 (2.4) | 1 (0.8) | 1 (2.5) | 0 (0.0) | 325 (7.0) |
| Infliximab biosimilar | 1040 (35.6) | 197 (17.9) | 31 (7.4) | 11 (8.5) | 3 (7.5) | 0 (0.0) | 1282 (27.8) |
| Adalimumab, n (%) | 747 (25.5) | 179 (16.3) | 21 (5.0) | 3 (2.3) | 3 (7.5) | 0 (0.0) | 953 (20.7) |
| Adalimumab originator | 486 (16.6) | 119 (10.8) | 11 (2.6) | 2 (1.5) | 0 (0.0) | 0 (0.0) | 618 (13.4) |
| Adalimumab biosimilar | 261 (8.9) | 60 (5.5) | 10 (2.4) | 1 (0.8) | 3 (7.5) | 0 (0.0) | 335 (7.3) |
| Golimumab, n (%) | 402 (13.8) | 67 (6.1) | 15 (3.6) | 3 (2.3) | 0 (0.0) | 1 (11.1) | 488 (10.6) |
| Vedolizumab, n (%) | 431 (14.8) | 479 (43.5) | 165 (39.4) | 10 (7.7) | 4 (10.0) | 1 (11.1) | 1090 (23.6) |
| Ustekinumab, n (%) | 22 (0.8) | 65 (5.9) | 84 (20.0) | 47 (36.2) | 17 (42.5) | 2 (22.2) | 238 (5.2) |
| Tofacitinib, n (%) | 14 (0.5) | 64 (5.8) | 93 (22.2) | 55 (42.3) | 12 (30.0) | 5 (55.6) | 243 (5.3) |
| Overall, n | 2920 | 1100 | 419 | 130 | 40 | 9 | 4619 |
| A. Crohn’s Disease | ||||
| 1st LoT. 4th iteration. | ||||
| Target | Variables | Results | ||
|
Adalimumab Infliximab Vedolizumab Ustekinumab |
Demographic variables
Clinical variables Treatment variables Hospitals Surgeries | Label | recall | precision |
| Adalimumab | 0.38 | 0.58 | ||
| Infliximab | 0.40 | 0.53 | ||
| Vedolizumab | 0.46 | 0.19 | ||
| Ustekinumab | 0.62 | 0.30 | ||
| Logistic regression | ||||
| 2nd LoT. 1st iteration. | ||||
|
Adalimumab biosimilar Adalimumab originator Infliximab biosimilar Infliximab originator Ustekinumab Vedolizumab |
Demographic variables
Clinical variables Treatment variables Hospitals Surgeries | Label | recall | precision |
| Adalimumab_biosim | 0.79 | 0.43 | ||
| Adalimumab_orig | 0.76 | 0.39 | ||
| Infliximab_biosim | 0.21 | 0.23 | ||
| Infliximab_original | 0.41 | 0.16 | ||
| Ustekinumab | 0.46 | 0.72 | ||
| Vedolizumab | 0.21 | 0.54 | ||
| Random Forest model | ||||
| B. Ulcerative Colitis | ||||
| 1st LoT, 3rd iteration. | ||||
|
Adalimumab Infliximab Other original a Vedolizumab |
Demographic variables
Clinical variables Treatment variables Hospitals Surgeries | Label | recall | precision |
| Adalimumab | 0.41 | 0.37 | ||
| Infliximab | 0.50 | 0.61 | ||
| Other_orig | 0.35 | 0.28 | ||
| Vedolizumab | 0.34 | 0.30 | ||
| XGBoost | ||||
| 2nd LoT, 1st iteration. | ||||
|
Adalimumab biosimilar Adalimumab original Infliximab biosimilar Infliximab original Golimumab Tofacitinib Vedolizumab |
Demographic variables
Clinical variables Treatment variables Hospitals Surgeries | Label | recall | precision |
| Adalimumab_biosim | 0.65 | 0.28 | ||
| Adalimumab_orig | 0.74 | 0.29 | ||
| Golimumab | 0.29 | 0.14 | ||
| Infliximab_biosim | 0.22 | 0.33 | ||
| Infliximab_original | 0.47 | 0.19 | ||
| Tofacitinib | 1.00 | 0.97 | ||
| Ustekinumab | 0.53 | 0.19 | ||
| Vedolizumab | 0.00 | 0.33 | ||
| Random Forest model | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Labrador, C.; Ricart, E.; Iborra, M.; Iglesias, E.; Martín-Arranz, M.D.; de Castro, L.; De Francisco, R.; García-Alonso, F.J.; Sanahuja, A.; Gargallo-Puyuelo, C.J.; et al. Trends in Targeted Therapy Usage in Inflammatory Bowel Disease: TRENDY Study of ENEIDA. Pharmaceutics 2024, 16, 629. https://doi.org/10.3390/pharmaceutics16050629
Gómez-Labrador C, Ricart E, Iborra M, Iglesias E, Martín-Arranz MD, de Castro L, De Francisco R, García-Alonso FJ, Sanahuja A, Gargallo-Puyuelo CJ, et al. Trends in Targeted Therapy Usage in Inflammatory Bowel Disease: TRENDY Study of ENEIDA. Pharmaceutics. 2024; 16(5):629. https://doi.org/10.3390/pharmaceutics16050629
Chicago/Turabian StyleGómez-Labrador, Celia, Elena Ricart, Marisa Iborra, Eva Iglesias, María Dolores Martín-Arranz, Luisa de Castro, Ruth De Francisco, Francisco Javier García-Alonso, Ana Sanahuja, Carla J. Gargallo-Puyuelo, and et al. 2024. "Trends in Targeted Therapy Usage in Inflammatory Bowel Disease: TRENDY Study of ENEIDA" Pharmaceutics 16, no. 5: 629. https://doi.org/10.3390/pharmaceutics16050629