Effects of Soils on Environmental Stability of Spent Mg-Based and Ca-Based Adsorbents Containing Arsenite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Synthetic As-Contaminated Water
2.3. Preparation of Spent Adsorbents
2.4. Soils
- Kuroboku soil (Ku) is Andosols-type distinguished by the chemical properties of aluminum such as aluminum–humus complexes; volcanic ash soils rich in organic components and particularly common in Japan.
- Yellow-brown forest soil (YF) is a slightly acidic and inorganic volcanic ash soil.
- Kanuma soil (Ka) is an acidic soil formed from weathered pumice. It is high in allophane, which is a type of clay mineral made of hydrated aluminosilicate.
- River sand (RS) is a sandy soil with a high silica content and an alkaline pH.
- Mountain sand (MS) is a sandy soil with a high iron content and an alkaline pH.
2.5. Leaching Tests (Shaking Tests)
3. Results
3.1. pH of Leachate
3.2. As Concentration in Leachate
3.3. Mg Concentration in Leachate
3.4. Ca Concentration in Leachate
3.5. Si Concentration in Leachate
3.6. Fe Concentration in Leachate
4. Discussion
4.1. As Leaching Ratio
4.2. Dissolved Forms of As in Leachate
4.3. Mg and Ca Leaching Ratios
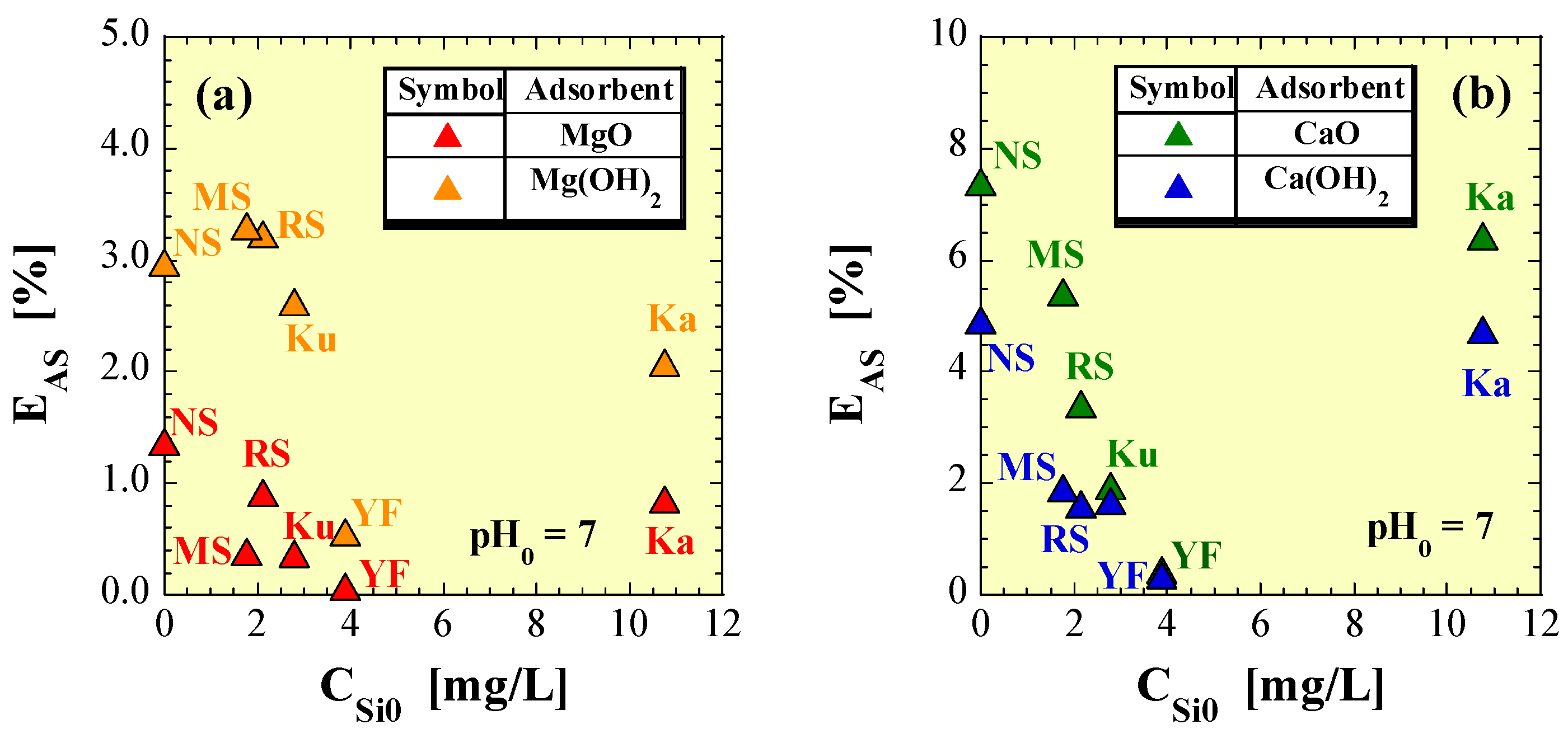
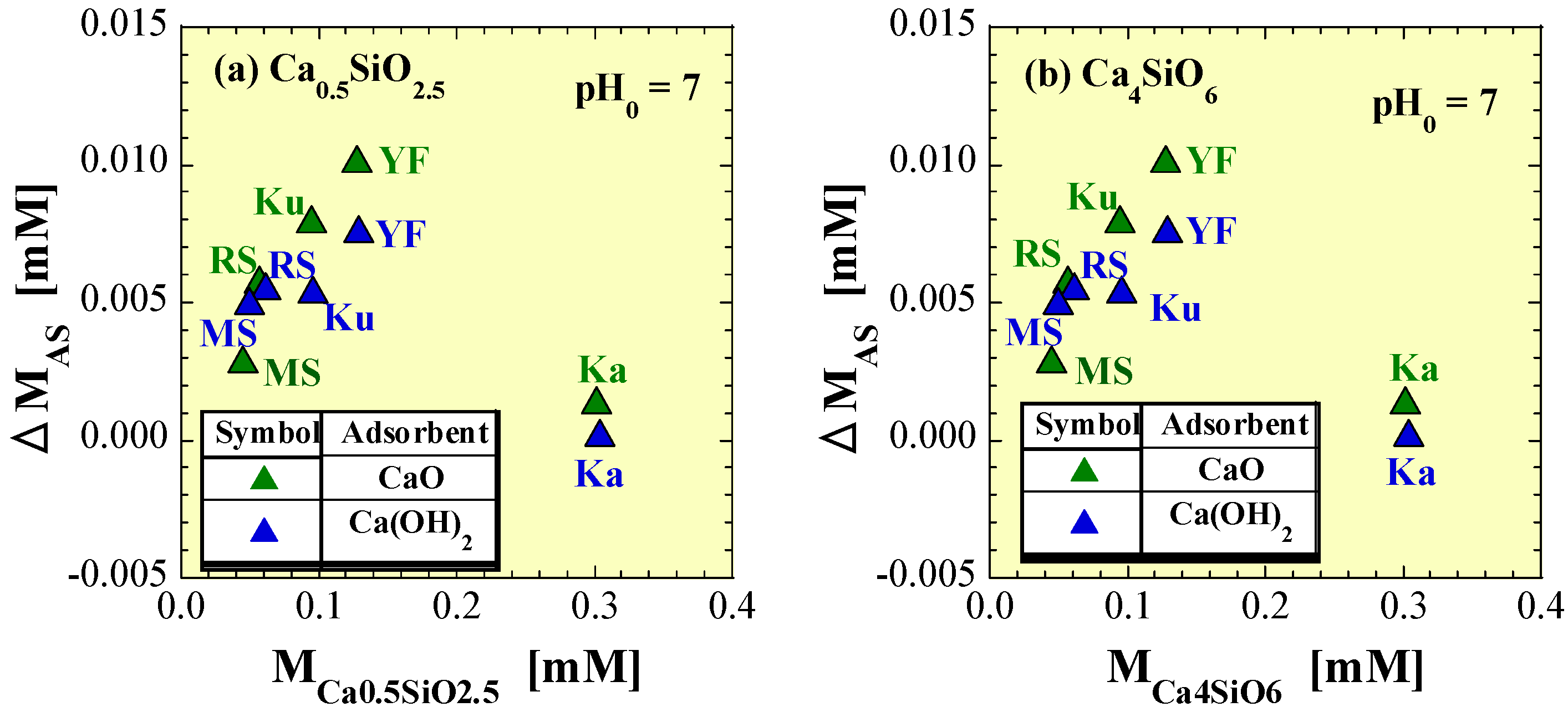
4.4. Effects of Silicic Acid Leached from Soils
4.5. Recommendations for Waste Disposal Considering Effects of Soil on Spent Adsorbents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; WHO: Tarxien, Malta, 2011; Arsenic; pp. 315–318. ISBN 978-92-4-154815-1. Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf (accessed on 21 February 2024).
- van Geen, A.; Zheng, Y.; Cheng, Z.; Aziz, Z.; Horneman, A.; Dhar, R.K.; Mailloux, B.; Stute, M.; Weinman, B.; Goodbred, S.; et al. A transect of groundwater and sediment properties in Araihazar, Bangladesh: Further evidence of decoupling between As and Fe mobilization. Chem. Geol. 2006, 228, 85–96. [Google Scholar] [CrossRef]
- Harvey, C.F.; Ashfaque, K.N.; Yu, W.; Badruzzaman, A.B.M.; Ali, M.A.; Oates, P.M.; Michael, H.A.; Neumann, R.B.; Beckie, R.; Islam, S.; et al. Groundwater dynamics and arsenic contamination in Bangladesh. Chem. Geol. 2006, 228, 112–136. [Google Scholar] [CrossRef]
- Senanayake, N.; Mukherji, A. Irrigating with arsenic contaminated groundwater in West Bengal and Bangladesh: A review of interventions for mitigating adverse health and crop outcomes. Agric. Water Manag. 2014, 135, 90–99. [Google Scholar] [CrossRef]
- Khan, K.M.; Parvez, F.; Zoeller, R.T.; Hocevar, B.A.; Kamendulis, L.M.; Rohlman, D.; Eunus, M.; Graziano, J. Thyroid hormones and neurobehavioral functions among adolescents chronically exposed to groundwater with geogenic arsenic in Bangladesh. Sci. Total Environ. 2019, 678, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.E.; Fahad, S.; Shao, Z.; Sarven, M.S.; Khan, I.A.; Alam, M.; Saeed, M.; Ullah, H.; Adnan, M.; Saud, S.; et al. Arsenic in a groundwater environment in Bangladesh. Occurrence and mobilization. J. Environ. Manag. 2020, 262, 110318. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Parvaiz, A.; Mushtaq, N.; Hussain, I.; Javed, T.; Rehman, H.U.; Farooqi, A. Characterization and role of derived dissolved organic matter on arsenic mobilization in alluvial aquifers of Punjab, Pakistan. Chemosphere 2020, 251, 126374. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Razeghi, N.; Zhang, Y.; Yang, M. Spatial distribution of arsenic in groundwater of Iran, a review. J. Geochem. Explor. 2019, 201, 88–98. [Google Scholar] [CrossRef]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; Hossain, M.A.; Chowdhury, U.K.; Biswas, B.K.; et al. Arsenic in groundwater of the Kolkata Municipal Corporation (KMC), India: Critical review and modes of mitigation. Chemosphere 2017, 180, 437–447. [Google Scholar] [CrossRef]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef]
- Bindal, S.; Singh, C.K. Predicting groundwater arsenic contamination: Regions at risk in highest populated state of India. Water Res. 2019, 159, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Diyabalanage, S.; Dissanayake, C.B. Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundw. Sustain. Dev. 2020, 10, 100359. [Google Scholar] [CrossRef]
- Hoang, T.H.; Bang, S.; Kim, K.W.; Nguyen, M.H.; Dang, D.M. Arsenic in groundwater and sediment in the Mekong River Delta, Vietnam. Environ. Poll. 2010, 158, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Trang, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and temporal evolution of groundwater arsenic contamination in the Red River Delta, Vietnam: Interplay of mobilisation and retardation processes. Sci. Total Environ. 2020, 717, 137143. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A.; Magnone, D.; Sültenfuß, J.; Chambers, L.; Bryant, C.; Boyce, A.J.; van Dongen, B.E.; Ballentine, C.J.; Sovann, C.; Uhlemann, S.; et al. Dual in-aquifer and near surface processes drive arsenic mobilization in Cambodian groundwaters. Sci. Total Environ. 2019, 659, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhang, F.; Zhang, E.; Wang, C.; Han, S.; Zheng, Y. Arsenic, fluoride and iodine in groundwater of China. J. Geochem. Explor. 2013, 135, 1–21. [Google Scholar] [CrossRef]
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, South-West China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Mariño, E.E.; Ávila, G.T.; Bhattacharya, P.; Schulz, C.J. The occurrence of arsenic and other trace elements in groundwaters of the southwestern Chaco-Pampean plain, Argentina. J. S. Am. Earth Sci. 2020, 100, 102547. [Google Scholar] [CrossRef]
- Aullón Alcaine, A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.P.; Mörth, C.M.; Sracek, O.; Ahmada, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671. [Google Scholar] [CrossRef]
- Machado, I.; Falchi, L.; Bühl, V.; Mañay, N. Arsenic levels in groundwater and its correlation with relevant inorganic parameters in Uruguay: A medical geology perspective. Sci. Total Environ. 2020, 721, 137787. [Google Scholar] [CrossRef] [PubMed]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.-F.; Cardona, A. Correlation of arsenic and fluoride in the groundwater for human consumption in a semiarid region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Rodríguez, R.; Lara Del Río, A.; Ruiz-Huerta, E.A.; Armienta, M.A.; Dávila-Harris, P.; Sen-Gupta, B.; Delgado-Rodríguez, O.; Del Angel Ríos, A.; Martínez-Villegas, N. Alluvial and gypsum karst geological transition favors spreading arsenic contamination in Matehuala, Mexico. Sci. Total Environ. 2020, 707, 135340. [Google Scholar] [CrossRef] [PubMed]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk. Sci. Total Environ. 2017, 584–585, 958–970. [Google Scholar] [CrossRef]
- Abiye, T.A.; Bhattacharya, P. Arsenic concentration in groundwater: Archetypal study from South Africa. Groundw. Sustain. Dev. 2019, 9, 100246. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alter-natives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U., Jr.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.Y.; Tran, T.; Lee, Y.H.; Nam, Y.I., II; Senanayake, G.; Kim, M.J. Selective removal of arsenic(V) from a molybdate plant liquor by precipitation of magnesium arsenate. Hydrometallurgy 2010, 104, 290–297. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Katsikini, M.; Paloura, E.C.; Bantsis, G.; Mitrakas, M. A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 2014, 265, 217–225. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Jia, Y.; Zhang, Y.X.; Liu, J.H.; Huang, X.J. Porous hierarchically micro-/nanostructured MgO: Morphology control and their excellent performance in As(III) and As(V) removal. J. Phys. Chem. C 2011, 115, 22242–22250. [Google Scholar] [CrossRef]
- Opiso, E.M.; Sato, T.; Morimoto, K.; Asai, A.; Anraku, S.; Numako, C.; Yoneda, T. Incorporation of arsenic during the formation of Mg-bearing minerals at alkaline condition. Miner. Eng. 2010, 23, 230–237. [Google Scholar] [CrossRef]
- Camacho, J.; Wee, H.Y.; Kramer, T.A.; Autenrieth, R. Arsenic stabilization on water treatment residuals by calcium addition. J. Hazard. Mater. 2009, 165, 599–603. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Concha-Lozano, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef]
- Olyaie, E.; Banejad, H.; Afkhami, A.; Rahmani, A.; Khodaveisi, J. Development of a cost-effective technique to remove the arsenic contamination from aqueous solutions by calcium peroxide nanoparticles. Sep. Purif. Technol. 2012, 95, 10–15. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Lo, S.-L.; Kuan, W.-H. High concentration of arsenate removal by electrocoagulation with calcium. Sep. Purif. Technol. 2014, 126, 7–14. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Takahashi, S. Environmental stability of spent magnesium-based and calcium-based arsenic adsorbents-Effects of soils. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2016, 72, 437–448. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of silicic acid on leaching behavior of arsenic from spent calcium-based adsorbents with arsenite. Sustainability 2021, 13, 12937. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of silicic acid on leaching behavior of arsenic from spent magnesium-based adsorbents containing arsenite. Sustainability 2022, 14, 4236. [Google Scholar] [CrossRef]
- The Chemical Society of Japan (CSJ). Kagaku Binran (Handbook of Chemistry), Pure Chemistry II, 4th ed.; Maruzen: Tokyo, Japan, 1993; p. 317. [Google Scholar]









| Adsorbent | P (%) | Dp50 (μm) | SBET (m2/g) | αMg (%) | αCa (%) |
|---|---|---|---|---|---|
| MgO | 98.0 | 1.54 | 4.3 | 59.1 | - |
| Mg(OH)2 | 99.9 | 4.13 | 22.0 | 40.6 | - |
| CaO | 99.6 | 19.6 | 2.7 | - | 71.2 |
| Ca(OH)2 | 98.9 | 41.7 | 14.3 | - | 53.5 |
| No. | As(Valence) | Adsorbent | WAD/V (g/L) | pH0 | CAS0 (mg/L) | CAS (mg/L) | CMg (mg/L) | CCa (mg/L) | RAS (%) | βMg (%) | βCa (%) | QAS (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) 1 | As(III) | MgO | 5.004 | 6.99 | 21.79 | 0.149 | 6.23 | - | 99.3 | 0.21 | - | 4.32 |
| (2) 1 | As(III) | Mg(OH)2 | 5.007 | 7.10 | 21.85 | 1.013 | 7.80 | - | 95.4 | 0.38 | - | 4.16 |
| (3) 2 | As(III) | CaO | 5.009 | 7.09 | 22.35 | 1.437 | - | 837 | 93.6 | - | 23.5 | 5.42 |
| (4) 2 | As(III) | Ca(OH)2 | 5.009 | 7.09 | 22.73 | 1.378 | - | 858 | 93.9 | - | 32.0 | 6.23 |
| Soil | Blank | MgO | Mg(OH)2 | CaO | Ca(OH)2 |
|---|---|---|---|---|---|
| NS | H3AsO3 | H2AsO3− | H2AsO3− | HAsO32−, H2AsO3− | HAsO32−, H2AsO3− |
| Ku | H3AsO3 | H3AsO3 | H3AsO3 | H3AsO3 | H3AsO3 |
| YF | H3AsO3 | H3AsO3 | H3AsO3 | H3AsO3, H2AsO3− | H3AsO3, H2AsO3− |
| Ka | H3AsO3 | H2AsO3−, H3AsO3 | H3AsO3 | H2AsO3−, H3AsO3 | H2AsO3−, H3AsO3 |
| RS | H3AsO3 | H2AsO3− | H2AsO3− | HAsO32−, H2AsO3− | HAsO32−, H2AsO3− |
| MS | H3AsO3 | H2AsO3− | H2AsO3− | HAsO32−, H2AsO3− | HAsO32−, H2AsO3− |
| As | Adsorbent | Ku | YF | Ka | RS | MS |
|---|---|---|---|---|---|---|
| As(III) | MgO | - | - | 0.09 | 59.1 | - |
| As(III) | Mg(OH)2 | - | - | 22.0 | 2.05 | 1.58 |
| As | Adsorbent | Ku | YF | Ka | RS | MS |
|---|---|---|---|---|---|---|
| As(III) | CaO | 203 | 152 | 67.1 | 77.6 | 59.2 |
| As(III) | Ca(OH)2 | 190 | 144 | 66.6 | 75.3 | 48.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita, H.; Morimoto, K.; Saito, T.; Hara, J. Effects of Soils on Environmental Stability of Spent Mg-Based and Ca-Based Adsorbents Containing Arsenite. Sustainability 2024, 16, 4008. https://doi.org/10.3390/su16104008
Sugita H, Morimoto K, Saito T, Hara J. Effects of Soils on Environmental Stability of Spent Mg-Based and Ca-Based Adsorbents Containing Arsenite. Sustainability. 2024; 16(10):4008. https://doi.org/10.3390/su16104008
Chicago/Turabian StyleSugita, Hajime, Kazuya Morimoto, Takeshi Saito, and Junko Hara. 2024. "Effects of Soils on Environmental Stability of Spent Mg-Based and Ca-Based Adsorbents Containing Arsenite" Sustainability 16, no. 10: 4008. https://doi.org/10.3390/su16104008





