Perovskite Oxide Catalysts for Enhanced CO2 Reduction: Embroidering Surface Decoration with Ni and Cu Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
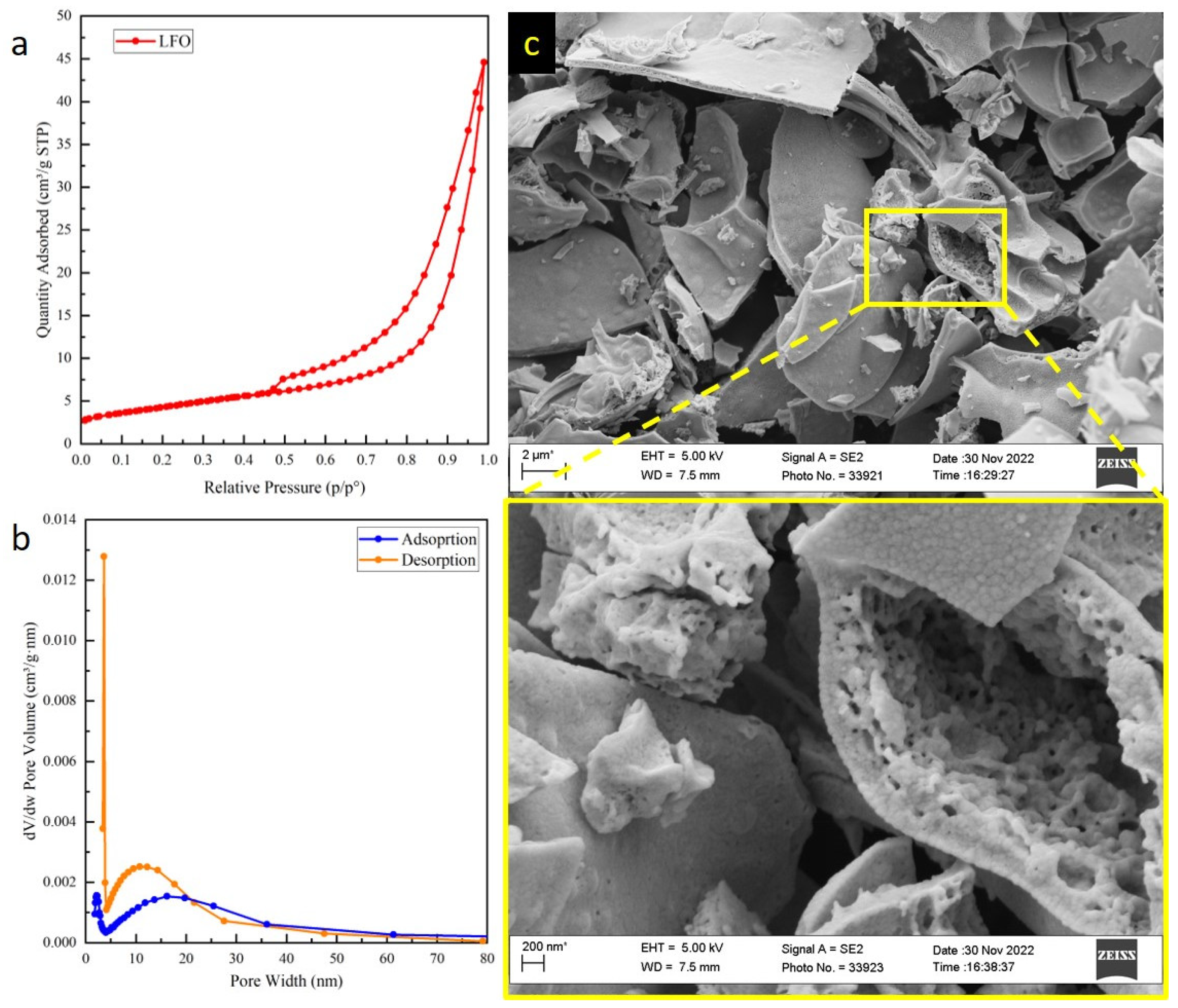
2.1. Structural and Morphological Characterizations
2.2. Bulk and Surface Composition
2.3. Catalytic Activity
2.4. Analysis on Spent Catalysts
3. Experimental Section
3.1. Synthesis of Catalysts
3.2. Characterizations
3.3. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.R.; Skea, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. IPCC, 2019 Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Hasegawa, T.; Sakurai, G.; Fujimori, S.; Takahashi, K.; Hijioka, Y.; Masui, T. Extreme Climate Events Increase Risk of Global Food Insecurity and Adaptation Needs. Nature Food 2021, 2, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture Methods, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and Challenges in CO2 Utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Takht Ravanchi, M.; Sahebdelfar, S. Catalytic Conversions of CO2 to Help Mitigate Climate Change: Recent Process Developments. Process Saf. Environ. Prot. 2021, 145, 172–194. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent Advances in Heterogeneous Photocatalytic CO2 Conversion to Solar Fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy Related CO2 Conversion and Utilization: Advanced Materials/Nanomaterials, Reaction Mechanisms and Technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Hightower, F.W.; White, A.H. Synthesis of Methane from Water Gas. Ind. Eng. Chem. 1928, 20, 10–15. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal Conversion of CO2 into CH4 with H2 over Group VIII Nanocatalysts: An Alternative Approach for Solar Fuel Production. Angew. Chem. Int. Ed. 2014, 53, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Song, C. Global Challenges and Strategies for Control, Conversion and Utilization of CO2 for Sustainable Development Involving Energy, Catalysis, Adsorption and Chemical Processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent Advances in Microbial CO2 Fixation and Conversion to Value-Added Products. Chem. Eng. J. 2020, 390, 124584. [Google Scholar] [CrossRef]
- Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts 2020, 10, 95. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.L.M.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on Inorganic Perovskites for Energy Applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Rizzato, L.; Cavazzani, J.; Osti, A.; Scavini, M.; Glisenti, A. Cu-Doped SrTiO3 Nanostructured Catalysts for CO2 Conversion into Solar Fuels Using Localised Surface Plasmon Resonance. Catalysts 2023, 13, 1377. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Ravichandran, G. Ferroelectric Perovskites for Electromechanical Actuation. Acta Mater. 2003, 51, 5941–5960. [Google Scholar] [CrossRef]
- Properties and Applications of Perovskite-Type Oxides; CRC Press: Boca Raton, FL, USA, 1993.
- Wang, Z.; Huang, H.; Li, G.; Yan, X.; Yu, Z.; Wang, K.; Wu, Y. Advances in Engineering Perovskite Oxides for Photochemical and Photoelectrochemical Water Splitting. Appl. Phys. Rev. 2021, 8, 021320. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Li, S.; Wang, D. Defect Engineering in Perovskite Oxide Thin Films. Chem. Commun. 2021, 57, 8402–8420. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The Role of Oxygen Vacancies of ABO3 Perovskite Oxides in the Oxygen Reduction Reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- Ruh, T.; Berkovec, D.; Schrenk, F.; Rameshan, C. Exsolution on Perovskite Oxides: Morphology and Anchorage of Nanoparticles. Chem. Commun. 2023, 59, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, K.; Wei, H.; Qin, Q.; Qi, W.; Yang, L.; Ruan, C.; Wu, Y. In Situ Formation of Oxygen Vacancy in Perovskite Sr0.95Ti0.8Nb0.1M0.1O3 (M = Mn, Cr) toward Efficient Carbon Dioxide Electrolysis. Sci. Rep. 2014, 4, 7082. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, M.; Jin, Y.; Zhou, M.; Mao, Y.; Sun, J.; Wang, W.; Song, Z. Low-Coordination Transition Metal Sites on Oxygen Vacancy Enriched Strontium Titanate-Based Perovskites Enable Highly Selective Photocatalytic CO2 Reduction to CH4. Appl. Catal. B 2024, 341, 123348. [Google Scholar] [CrossRef]
- Kozokaro, V.F.; Addo, P.K.; Ansari, H.M.; Birss, V.I.; Toroker, M.C. Optimal Oxygen Vacancy Concentration for CO2 Reduction in LSFCR Perovskite: A Combined Density Functional Theory and Thermogravimetric Analysis Measurement Study. J. Phys. Chem. C 2020, 124, 27453–27466. [Google Scholar] [CrossRef]
- Feng, C.; Gao, Q.; Xiong, G.; Chen, Y.; Pan, Y.; Fei, Z.; Li, Y.; Lu, Y.; Liu, C.; Liu, Y. Defect Engineering Technique for the Fabrication of LaCoO3 Perovskite Catalyst via Urea Treatment for Total Oxidation of Propane. Appl. Catal. B 2022, 304, 121005. [Google Scholar] [CrossRef]
- Lindenthal, L.; Popovic, J.; Rameshan, R.; Huber, J.; Schrenk, F.; Ruh, T.; Nenning, A.; Löffler, S.; Opitz, A.K.; Rameshan, C. Novel Perovskite Catalysts for CO2 Utilization—Exsolution Enhanced Reverse Water-Gas Shift Activity. Appl. Catal. B 2021, 292, 120183. [Google Scholar] [CrossRef]
- Liu, J.; Kim, J.K.; Wang, Y.; Kim, H.; Belotti, A.; Koo, B.; Wang, Z.; Jung, W.C.; Ciucci, F. Understanding and Mitigating A-Site Surface Enrichment in Ba-Containing Perovskites: A Combined Computational and Experimental Study of BaFeO3. Energy Environ. Sci. 2022, 15, 4069–4082. [Google Scholar] [CrossRef]
- Shyamal, S.; Dutta, S.K.; Das, T.; Sen, S.; Chakraborty, S.; Pradhan, N. Facets and Defects in Perovskite Nanocrystals for Photocatalytic CO2Reduction. J. Phys. Chem. Lett. 2020, 11, 3608–3614. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hong, J.; Huang, J.; Chen, W.; Segre, C.U.; Suenaga, K.; Zhao, W.; Huang, F.; Wang, J. Surface Decoration Accelerates the Hydrogen Evolution Kinetics of a Perovskite Oxide in Alkaline Solution. Energy Environ. Sci. 2020, 13, 4249–4257. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Tian, J.; Yan, B. Cu/LaFeO3 as an Efficient and Stable Catalyst for CO2 Reduction: Exploring Synergistic Effect between Cu and LaFeO3. AIChE J. 2022, 68, e17640. [Google Scholar] [CrossRef]
- Schrenk, F.; Lindenthal, L.; Pacholik, G.; Navratil, T.; Berger, T.M.; Drexler, H.; Rameshan, R.; Ruh, T.; Föttinger, K.; Rameshan, C. Perovskite-Type Oxide Catalysts in CO2 Utilization: A Principal Study of Novel Cu-Doped Perovskites for Methanol Synthesis. Compounds 2022, 2, 378–387. [Google Scholar] [CrossRef]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T.S. Exsolution of Fe–Ni Alloy Nanoparticles from (La,Sr)(Cr,Fe,Ni)O3 Perovskites as Potential Oxygen Transport Membrane Catalysts for Methane Reforming. J. Mater. Chem. A Mater. 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Isupova, L.A.; Nadeev, A.N.; Prosvirin, I.P.; Dovlitova, L.S. Insights into the Reactivity of La1-XSrxMnO3 (x = 0 ÷ 0.7) in High Temperature N2O Decomposition. Catal. Lett. 2011, 141, 322–331. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and Applications of Nanoporous Perovskite Metal Oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.L.; Shi, P.C.; Huang, Y.B.; Cao, R. Atomically Dispersed Ni Species on N-Doped Carbon Nanotubes for Electroreduction of CO2 with Nearly 100% CO Selectivity. Appl. Catal. B 2020, 271, 118929. [Google Scholar] [CrossRef]
- Li, H.; Wei, P.; Gao, D.; Wang, G. In Situ Raman Spectroscopy Studies for Electrochemical CO2 Reduction over Cu Catalysts. Curr. Opin. Green Sustain. Chem. 2022, 34, 100589. [Google Scholar] [CrossRef]
- Carollo, G.; Garbujo, A.; Bedon, A.; Ferri, D.; Natile, M.M.; Glisenti, A. Cu/CGO Cermet Based Electrodes for Symmetric and Reversible Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 13652–13658. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, L.; Liu, H.; Cao, Z.; Yu, W.; Zhu, X.; Yang, W. Alkaline-Earth Elements (Ca, Sr and Ba) Doped LaFeO3-δ Cathodes for CO2 Electroreduction. J. Power Sources 2019, 443, 227268. [Google Scholar] [CrossRef]
- Ibarra-Rodriguez, L.I.; Garay-Rodríguez, L.F.; Torres-Martínez, L.M. Photocatalytic Reduction of CO2 over LaMO3 (M: Fe, Co, Mn) /CuxO Films. Mater. Sci. Semicond. Process. 2022, 139, 106328. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Schön, A.; Dujardin, C.; Dacquin, J.P.; Granger, P. Enhancing catalytic activity of perovskite-based catalysts in three-way catalysis by surface composition optimisation. Catal. Today 2015, 258, 543–548. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Khodadadi, A.A.; Mortazavi, Y. Self-regenerative function of Cu in LaMnCu0. 1O3 catalyst: Towards noble metal-free intelligent perovskites for automotive exhaust gas treatment. Appl. Catal. A Gen. 2020, 602, 117702. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Hu, N.; Shi, W.; Wang, F. Alloying Ni–Cu nanoparticles encapsulated in SiO2 nanospheres for synergistic catalysts in CO2 reforming with methane reaction. ACS Appl. Mater. Interfaces 2022, 14, 23487–23495. [Google Scholar] [CrossRef]








| Sample | La (at%) * | Fe (at%) * | Ni (at%) * | Cu (at%) * | La/Fe | Ni/Fe | Cu/Fe | |
|---|---|---|---|---|---|---|---|---|
| LFO | EDX | 49.5 | 50.5 | - | - | 0.98 | - | - |
| XPS as-prep. | 44.2 | 55.8 | - | - | 0.79 | - | - | |
| (nominal) | (44.5) | (55.5) | - | - | 0.90 | - | - | |
| Ni/LFO | EDX | 48.1 | 48.4 | 3.5 | - | 0.99 | 0.07 | - |
| XPS as-prep. | 45.6 | 47.6 | 6.8 | - | 0.96 | 0.14 | - | |
| XPS reduced | 52.7 | 41.9 | 5.4 | - | 1.26 | 0.13 | - | |
| (nominal) | (43.8) | (48.6) | (7.6) | - | (0.90) | (0.16) | - | |
| Cu/LFO | EDX | 47.8 | 49.3 | - | 2.9 | 0.97 | - | 0.06 |
| XPS as-prep. | 35.6 | 46.6 | - | 17.8 | 0.76 | - | 0.38 | |
| XPS reduced | 41.4 | 36.7 | - | 21.9 | 1.13 | - | 0.60 | |
| (nominal) | (44.0) | (48.9) | - | (7.1) | (0.90) | - | (0.15) | |
| Ni-Cu/LFO | EDX | 45.0 | 48.9 | 3.0 | 3.1 | 0.92 | 0.06 | 0.06 |
| XPS as-prep. | 38.8 | 50.0 | 1.5 | 9.7 | 0.78 | 0.03 | 0.19 | |
| XPS reduced | 40.0 | 37.6 | 4.8 | 17.6 | 1.06 | 0.13 | 0.47 | |
| (nominal) | (43.9) | (48.8) | (3.8) | (3.5) | (0.90) | (0.08) | (0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osti, A.; Rizzato, L.; Cavazzani, J.; Meneghello, A.; Glisenti, A. Perovskite Oxide Catalysts for Enhanced CO2 Reduction: Embroidering Surface Decoration with Ni and Cu Nanoparticles. Catalysts 2024, 14, 313. https://doi.org/10.3390/catal14050313
Osti A, Rizzato L, Cavazzani J, Meneghello A, Glisenti A. Perovskite Oxide Catalysts for Enhanced CO2 Reduction: Embroidering Surface Decoration with Ni and Cu Nanoparticles. Catalysts. 2024; 14(5):313. https://doi.org/10.3390/catal14050313
Chicago/Turabian StyleOsti, Andrea, Lorenzo Rizzato, Jonathan Cavazzani, Ambra Meneghello, and Antonella Glisenti. 2024. "Perovskite Oxide Catalysts for Enhanced CO2 Reduction: Embroidering Surface Decoration with Ni and Cu Nanoparticles" Catalysts 14, no. 5: 313. https://doi.org/10.3390/catal14050313







