Constructing Self-Healing Polydimethylsiloxane through Molecular Structure Design and Metal Ion Bonding
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Synthesis of Py-PDMS
2.3. Preparation of Eu3+-Py-PDMS Complex
2.4. Characterization
3. Results and Discussion
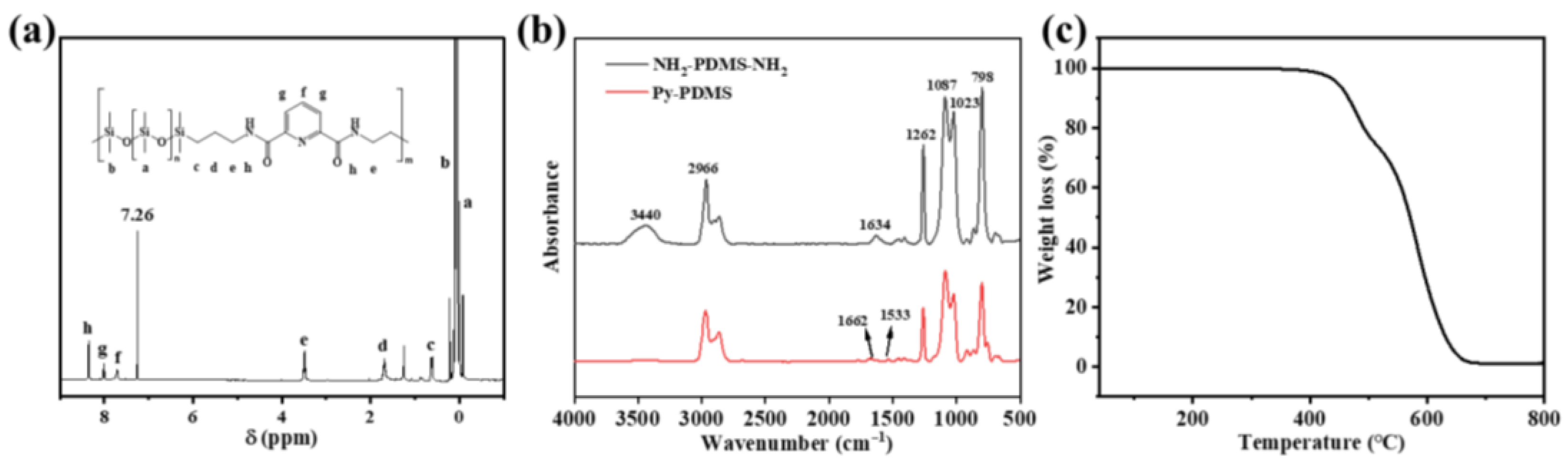
3.1. Molecular Structure of Py-PDMS
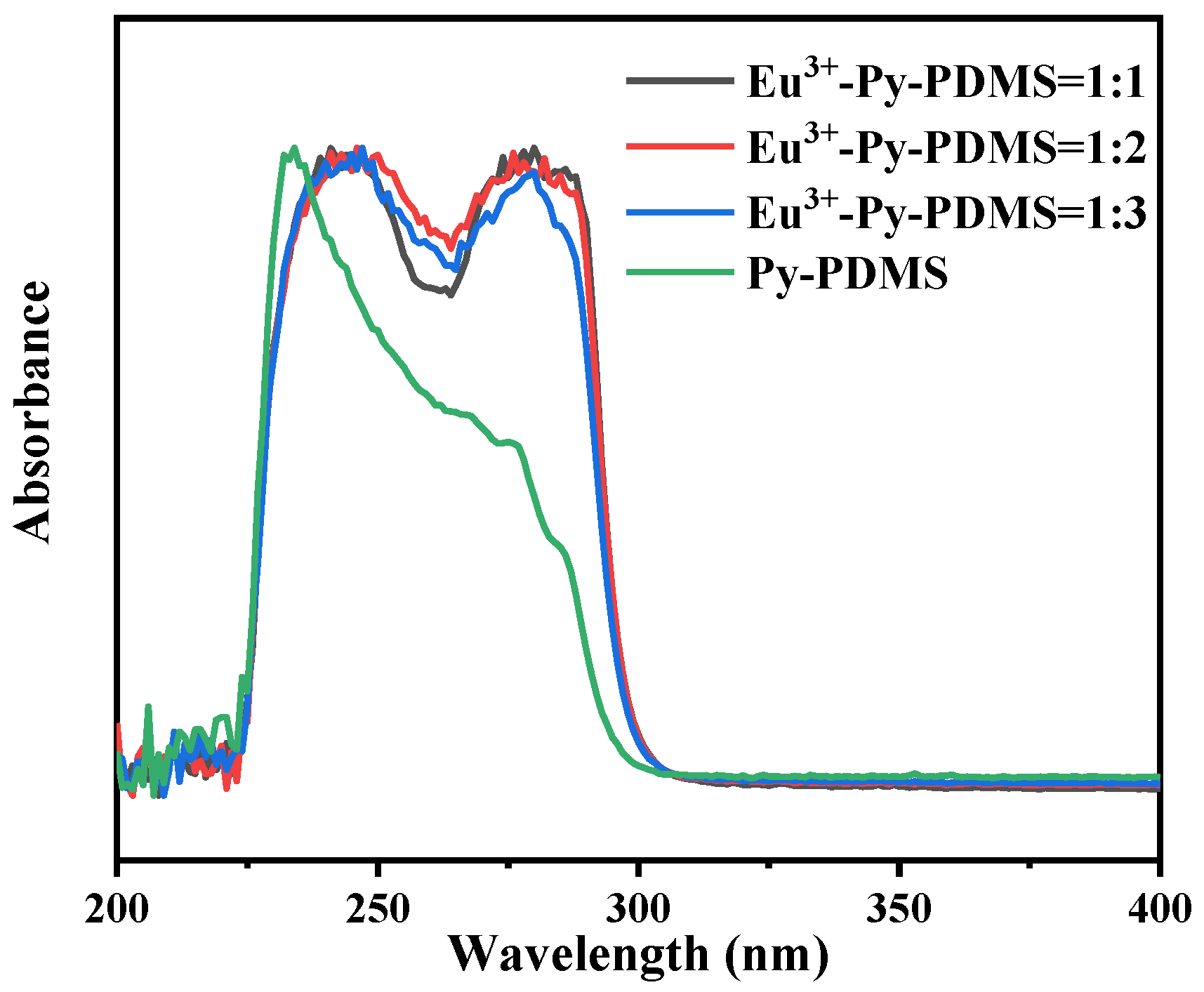
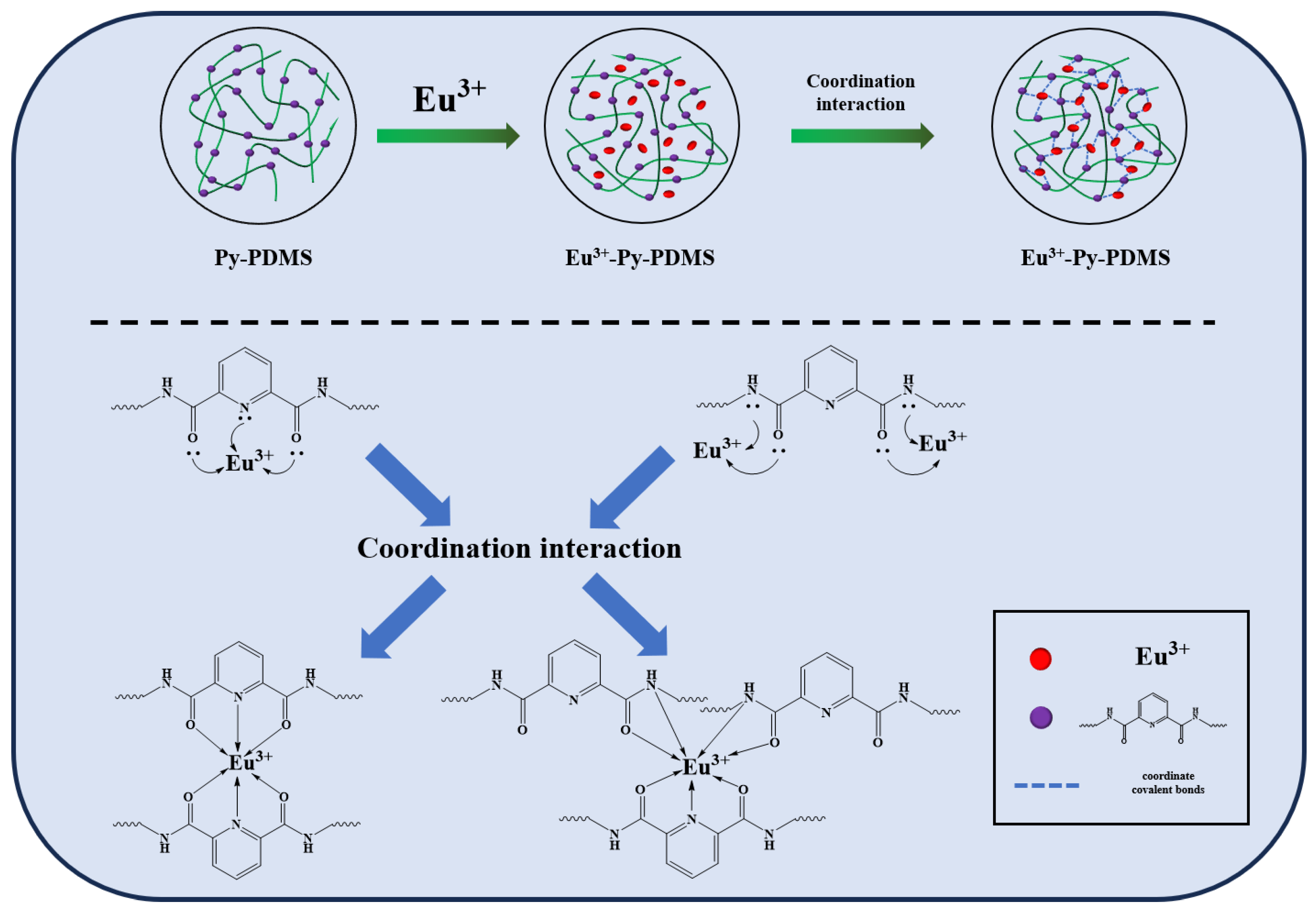
3.2. Structure Characterization of Eu3+-Py-PDMS
3.3. Mechanical Properties of Eu3+-Py-PDMS Complex
3.4. Self-Healing Properties of Eu3+-Py-PDMS Complex
3.5. Gas Barrier Properties of Eu3+-Py-PDMS Complex
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane composites characterization and its applications: A review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Fortenbaugh, R.J.; Lear, B.J. On-demand curing of polydimethylsiloxane (PDMS) using the photothermal effect of gold nanoparticles. Nanoscale 2017, 9, 8555–8559. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, Y.; Long, L.; Zhou, S.; Yan, L.; Zhang, J.; Zou, H. Fabrication of Highly Thermally Resistant and Self-Healing Polysiloxane Elastomers by Constructing Covalent and Reversible Networks. Macromol. Rapid Commun. 2023, 44, 2300191. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, C.; Li, Z.; Zheng, J. Facile Preparation of Polydimethylsiloxane Elastomer with Self-Healing Property and Remoldability Based on Diels-Alder Chemistry. Macromol. Mater. Eng. 2018, 303, 1800089. [Google Scholar] [CrossRef]
- Feng, Z.; Yu, B.; Hu, J.; Zuo, H.; Li, J.; Sun, H.; Ning, N.; Tian, M.; Zhang, L. Multifunctional vitrimer-like polydimethylsiloxane (PDMS): Recyclable, self-healable, and water-driven malleable covalent networks based on dynamic imine bond. Ind. Eng. Chem. Res. 2019, 58, 1212–1221. [Google Scholar] [CrossRef]
- Xiang, H.P.; Rong, M.Z.; Zhang, M.Q. A facile method for imparting sunlight driven catalyst-free self-healability and recyclability to commercial silicone elastomer. Polymer 2017, 108, 339–347. [Google Scholar] [CrossRef]
- Lai, J.C.; Mei, J.F.; Jia, X.Y.; Li, C.H.; You, X.Z.; Bao, Z. A stiff and healable polymer based on dynamic-covalent boroxine bonds. Adv. Mater. 2016, 28, 8277–8282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Ruan, Y.-B.; Zhang, B.-Q.; Qiao, X.; Deng, G.; Chen, Y.; Liu, C.-Y. A self-healing PDMS elastomer based on acylhydrazone groups and the role of hydrogen bonds. Polymer 2017, 120, 189–196. [Google Scholar] [CrossRef]
- Zhao, K.; Lv, C.; Zheng, J. A robust mechanochromic self-healing poly (dimethylsiloxane) elastomer. Sci. China Technol. Sci. 2020, 63, 740–747. [Google Scholar] [CrossRef]
- Yan, H.; Dai, S.; Chen, Y.; Ding, J.; Yuan, N. A high stretchable and self–healing silicone rubber with double reversible bonds. ChemistrySelect 2019, 4, 10719–10725. [Google Scholar] [CrossRef]
- Cao, P.F.; Li, B.; Hong, T.; Townsend, J.; Qiang, Z.; Xing, K.; Vogiatzis, K.D.; Wang, Y.; Mays, J.W.; Sokolov, A.P.; et al. Superstretchable, Self-Healing Polymeric Elastomers with Tunable Properties. Adv. Funct. Mater. 2018, 28, 1800741. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Y.; Wang, L.; Zhang, A. The synthesis and characterization of supramolecular elastomers based on linear carboxyl-terminated polydimethylsiloxane oligomers. Polym. Chem. 2014, 5, 153–160. [Google Scholar] [CrossRef]
- Madsen, F.B.; Yu, L.; Skov, A.L. Self-healing, high-permittivity silicone dielectric elastomer. ACS Macro Lett. 2016, 5, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, N.; Yan, D.; Song, J.; Fu, W.; Li, Z. Design of a mechanically strong and highly stretchable thermoplastic silicone elastomer based on coulombic interactions. J. Mater. Chem. A 2020, 8, 5943–5951. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Hu, R.; Lv, C.; Zheng, J. A highly ionic conductive, healable, and adhesive polysiloxane-supported ionogel. Macromol. Rapid Commun. 2019, 40, 1800776. [Google Scholar] [CrossRef] [PubMed]
- Boumezgane, O.; Suriano, R.; Fedel, M.; Tonelli, C.; Deflorian, F.; Turri, S. Self-healing epoxy coatings with microencapsulated ionic PDMS oligomers for corrosion protection based on supramolecular acid-base interactions. Prog. Org. Coat. 2022, 162, 106558. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W.; Feng, H.; Sun, T.; Ma, C.; Cao, L.; Qin, X.; Lei, Y.; Piao, J.; Feng, C. Photothermal nanofiller-based polydimethylsiloxane anticorrosion coating with multiple cyclic self-healing and long-term self-healing performance. Chem. Eng. J. 2022, 446, 137077. [Google Scholar] [CrossRef]
- Wang, D.-P.; Lai, J.-C.; Lai, H.-Y.; Mo, S.-R.; Zeng, K.-Y.; Li, C.-H.; Zuo, J.-L. Distinct mechanical and self-healing properties in two polydimethylsiloxane coordination polymers with fine-tuned bond strength. Inorg. Chem. 2018, 57, 3232–3242. [Google Scholar] [CrossRef]
- Jia, X.-Y.; Mei, J.-F.; Lai, J.-C.; Li, C.-H.; You, X.-Z. A self-healing PDMS polymer with solvatochromic properties. Chem. Commun. 2015, 51, 8928–8930. [Google Scholar] [CrossRef]
- Xu, C.; Wu, W.; Zheng, Z.; Nie, J.; Chen, Y. Strengthened, conductivity-tunable, and low solvent-sensitive flexible conductive rubber films with a Zn2+-crosslinked one-body segregated network. Compos. Sci. Technol. 2021, 203, 108606. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Liu, S.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. A supramolecular silicone dielectric elastomer with a high dielectric constant and fast and highly efficient self-healing under mild conditions. J. Mater. Chem. A 2020, 8, 23330–23343. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Xie, L.; Wang, W.; Wang, L.; Zhang, C.; Li, L.; Feng, S. Mechanically Strong, Autonomous Self-Healing, and Fully Recyclable Silicone Coordination Elastomers with Unique Photoluminescent Properties. Macromol. Rapid Commun. 2021, 42, 2100519. [Google Scholar] [CrossRef]
- Baranovskaya, V.; Karpov, Y.A.; Petrova, K.; Korotkova, N. Actual trends in the application of rare-earth metals and their compounds in the production of magnetic and luminescent materials: A review. Russ. J. Non-Ferr. Met. 2021, 62, 10–31. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Zhang, Z.; Liu, Y.; Epaarachchi, J.; Fang, Z.; Fang, L.; Lu, C.; Xu, Z. Effects of Ligands in Rare Earth Complex on Properties, Functions, and Intelligent Behaviors of Polyurea–Urethane Composites. Polymers 2022, 14, 2098. [Google Scholar] [CrossRef] [PubMed]
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 2782-1:2022; Rubber, Vulcanized or Thermoplastic Determination of Permeability to Gases Part 1: Differential-Pressure Methods. International Organization for Standardization: Geneva, Switzerland, 2022.
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, mid-infrared, near-infrared and ultraviolet–visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Ignatova, N.A.; Kirichenko, S.O.; Novikov, A.S.; Kryukova, M.A.; Kukushkin, V.Y.; Islamova, R.M. Structural Features of Polymer Ligand Environments Dramatically Affect the Mechanical and Room-Temperature Self-Healing Properties of Cobalt (II)-Incorporating Polysiloxanes. Organometallics 2021, 40, 2750–2760. [Google Scholar] [CrossRef]
- Rao, Y.-L.; Chortos, A.; Pfattner, R.; Lissel, F.; Chiu, Y.-C.; Feig, V.; Xu, J.; Kurosawa, T.; Gu, X.; Wang, C.; et al. Stretchable Self-Healing Polymeric Dielectrics Cross-Linked Through Metal–Ligand Coordination. J. Am. Chem. Soc. 2016, 138, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.; Xu, L.; Zhang, X.; Zhang, A.; Xu, Y. A highly stretchable, transparent, notch-insensitive self-healing elastomer for coating. J. Mater. Chem. C 2020, 8, 2043–2053. [Google Scholar] [CrossRef]
- Mei, J.F.; Jia, X.Y.; Lai, J.C.; Sun, Y.; Li, C.H.; Wu, J.H.; Cao, Y.; You, X.Z.; Bao, Z. A Highly Stretchable and Autonomous Self-Healing Polymer Based on Combination of Pt...Pt and pi-pi Interactions. Macromol. Rapid Commun. 2016, 37, 1667–1675. [Google Scholar] [CrossRef]
- Deriabin, K.V.; Ignatova, N.A.; Kirichenko, S.O.; Novikov, A.S.; Islamova, R.M. Nickel(II)-pyridinedicarboxamide-co-polydimethylsiloxane complexes as elastic self-healing silicone materials with reversible coordination. Polymer 2021, 212, 123119. [Google Scholar] [CrossRef]
- Oh, I.; Jeon, S.I.; Chung, I.J.; Ahn, C.-H. Self-Healable Dielectric Polydimethylsiloxane Composite Based on Zinc-Imidazole Coordination Bond. Macromol. Res. 2019, 27, 435–443. [Google Scholar] [CrossRef]
- Shan, Y.; Zhou, Z.; Bai, H.; Wang, T.; Liu, L.; Zhao, X.; Huang, Y. Recovery of the self-cleaning property of silicon elastomers utilizing the concept of reversible coordination bonds. Soft Matter 2020, 16, 8473–8481. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Huang, J.; Yang, S. Robust, Stretchable, and Self-Healable Supramolecular Elastomers Synergistically Cross-Linked by Hydrogen Bonds and Coordination Bonds. ACS Appl. Mater. Interfaces 2019, 11, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Yu, S.; Zuo, H.; Xu, X.; Ning, N.; Yu, B.; Zhang, L.; Tian, M. Self-Healable Silicone Elastomer Based on the Synergistic Effect of the Coordination and Ionic Bonds. ACS Appl. Polym. Mater. 2021, 3, 2667–2677. [Google Scholar] [CrossRef]








| Samples | Self-Healing Condition | Self-Healing Efficiency | Ref. |
|---|---|---|---|
| PDMS-NNN-Zn | 12 h at 25 °C | 91.3% | [19] |
| PDMS-PtL | 12 h at room temperature | 100% | [32] |
| Ni-Py-PDMS | 72 h at room temperature | 90.0% | [33] |
| IMZ-PDMS | 31 h at 25 °C | 98% | [34] |
| Zn-IC-PDMS | 24 h at room temperature | 96% | [35] |
| PDMS−TDI−Al | 36 h at room temperature | 90% | [36] |
| Fe3+-Hpdca-PDMS | 48 h at 25 °C | 90.0% | [37] |
| PDMS-COOH/Al/NH2 | 8 h at 60 °C | 80% | [38] |
| Eu3+-Py-PDMS | 4 h at 20 °C | 79% | This study |
| Eu3+-Py-PDMS | 4 h at 60 °C | Nearly 100% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Zhou, Y.; Zhao, Z.; Wang, Q.; Chu, L.; Wen, S. Constructing Self-Healing Polydimethylsiloxane through Molecular Structure Design and Metal Ion Bonding. Polymers 2024, 16, 1309. https://doi.org/10.3390/polym16101309
Qiu L, Zhou Y, Zhao Z, Wang Q, Chu L, Wen S. Constructing Self-Healing Polydimethylsiloxane through Molecular Structure Design and Metal Ion Bonding. Polymers. 2024; 16(10):1309. https://doi.org/10.3390/polym16101309
Chicago/Turabian StyleQiu, Lvchao, Yutong Zhou, Zhoufeng Zhao, Qi Wang, Lijun Chu, and Shipeng Wen. 2024. "Constructing Self-Healing Polydimethylsiloxane through Molecular Structure Design and Metal Ion Bonding" Polymers 16, no. 10: 1309. https://doi.org/10.3390/polym16101309






