Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.1.1. GelMA Lyophilizate
2.1.2. Culture Medium and PBS
2.1.3. Reconstitution Agent P
2.1.4. Sodium Hydroxide (NaOH) and Hydrochloric Acid (HCl)
2.1.5. Cells
2.2. Equipment
- Biological binocular microscope AE-20, MOTIC (Barcelona, Spain).
- Epifluorescence optical microscope Nikon Eclipse 80i (Tokyo, Japan).
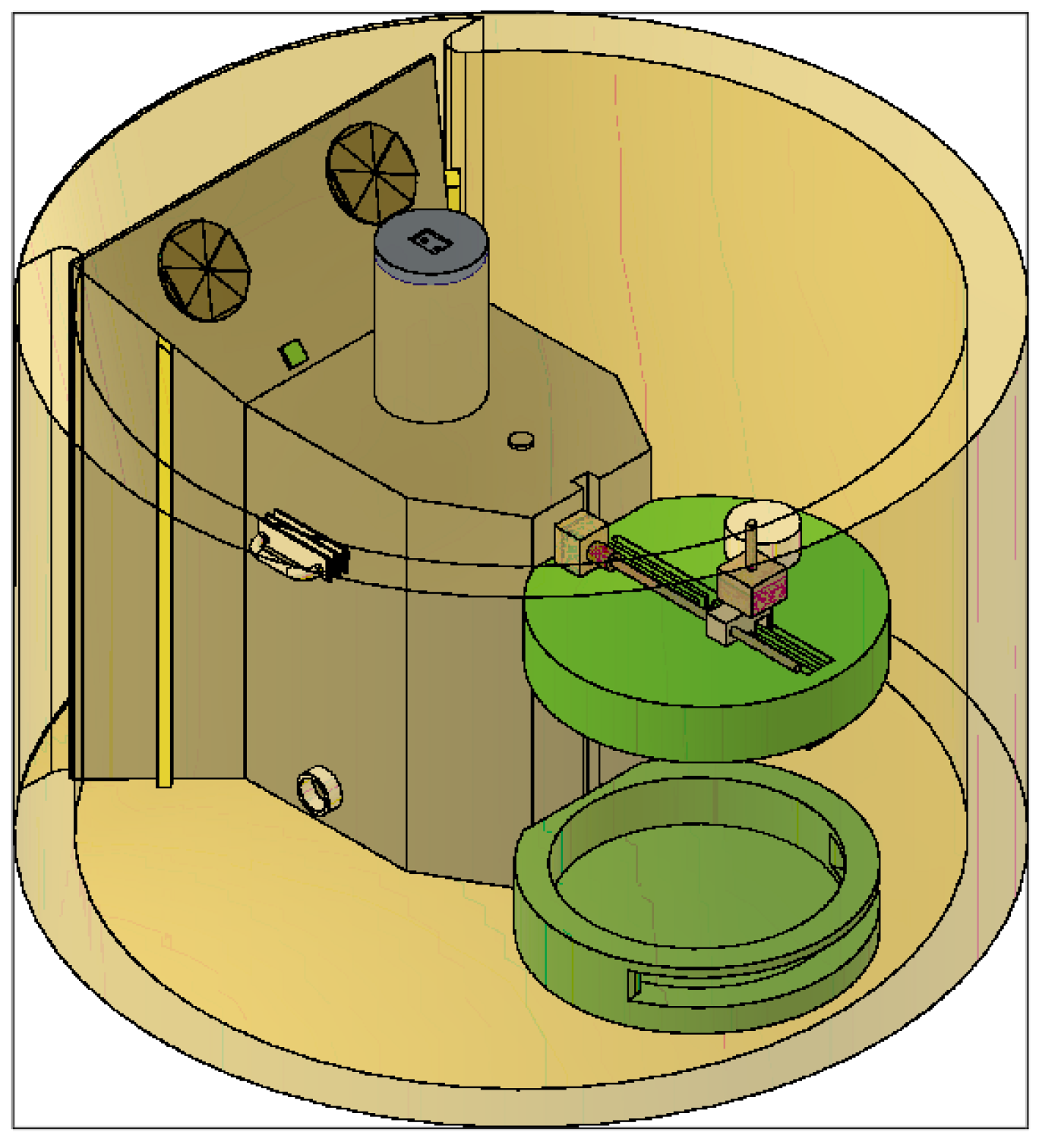
- Test device for the optimisation of 3D bioprinting, specially designed for the testing of different parameters defining the printability of hydrogels. Patent number EN 1 303 662 U.
- Eppendorf Galaxy 48R CO2 Incubator.
- Vertical laminar airflow cabinet Mini V/PCR.
- Cellink BIO X Bioprinter from CELLINK.
2.3. Method
2.3.1. Preparation of Hydrogels
2.3.2. Proportion of Hydrogels
2.3.3. Rheological Characterisation
2.3.4. Characterisation of Printability
2.3.5. Viability Assay
3. Results
3.1. Characterisation of the GelMA 100% Hydrogel
3.2. Rheological Characterisation of the Culture Medium and Buffers
3.3. Rheological Characterisation of GelMA Hydrogel with Culture Medium in Different Proportions
Analysis of the Storage Modulus as a Function of Temperature
- Zone 1: at low temperatures (<32 °C), we see that G′ is higher than G″, so the ink is more elastic and approaches a gel state or behaviour.
- Zone 2: at around 32 °C, we observe that G′ intersects with G″, which marks the gelation temperature and indicates that the hydrogel gels have decreasing temperature.
- Zone 3: at temperatures above 32 °C, G′ equals G″ and is even slightly lower, again presenting a gel state behaviour.
3.4. Characterisation of Printability
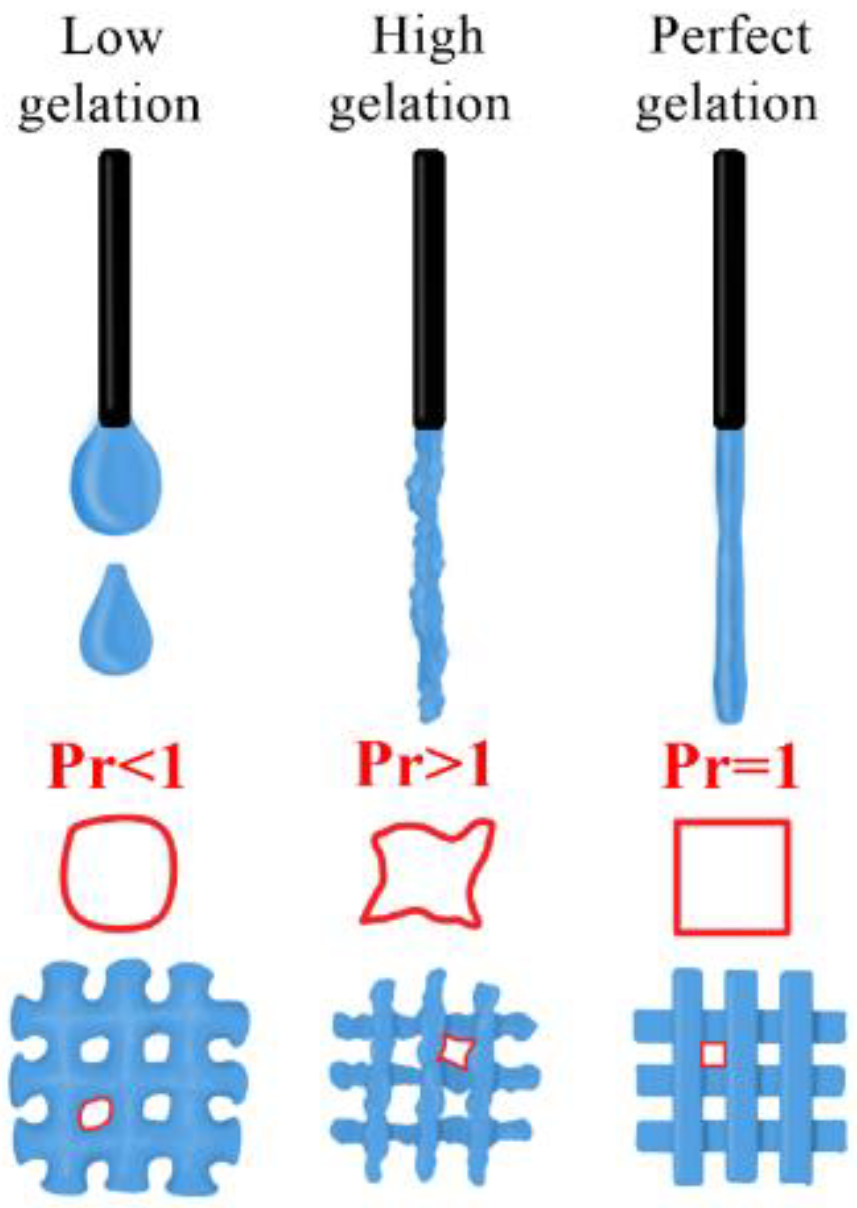
3.4.1. Quantitative Assessment of the Gelation State and Printing Grid Test
3.4.2. Strand Collapse Test
3.5. Toxicological Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2020, 9, 535–573. [Google Scholar] [CrossRef]
- Fazal, F.; Melchels, F.P.; McCormack, A.; Silva, A.F.; Callanan, A.; Koutsos, V.; Radacsi, N. A vertical additive-lathe printing system for the fabrication of tubular constructs using gelatin methacryloyl hydrogel. J. Mech. Behav. Biomed. Mater. 2023, 139, 105665. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Lee, J.W.; Cho, D.-W. Extrusion Bioprinting. In Essentials of 3D Biofabrication and Translation; Atala, A., Yoo, J.J., Eds.; Academic Press: Winston-Salem, NC, USA, 2015; pp. 123–152. [Google Scholar] [CrossRef]
- Rizwan, M.; Baker, A.E.G.; Shoichet, M.S. Designing Hydrogels for 3D Cell Culture Using Dynamic Covalent Crosslinking. Adv. Heal. Mater. 2021, 10, 2100234. [Google Scholar] [CrossRef]
- Kumar, A.; Placone, J.K.; Engler, A.J. Understanding the extracellular forces that determine cell fate and maintenance. Development 2017, 144, 4261–4270. [Google Scholar] [CrossRef]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Gowdini, M.; Khademi, A.; Dehghani, M.; Latifi, M.; Alsaadi, N.; Hemati, M.; Mohammadi, R.; Saber-Samandari, S.; Toghraie, D.; et al. Bioprinting of three-dimensional scaffold based on alginate-gelatin as soft and hard tissue regeneration. J. Mater. Res. Technol. 2021, 14, 2853–2864. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Daly, A.C.; E Critchley, S.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, V. Study of the Feasibility of a Soft Gelatin Methacryloyl-Based Template to Develop Cell-Derived Matrices for Screening Patients with Congenital Muscular Dystrophies. Doctoral Dissertation, Universitat de Barcelona, Barcelona, Spain, 2021. [Google Scholar]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Gasek, N.; Hwang, J.; Weiss, D.J.; Lee, P.C. Effect of temperature on gelation and cross-linking of gelatin methacryloyl for biomedical applications. Phys. Fluids 2020, 32, 033102. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, N.; Wei, W.; Hu, X.; Tan, Y.; Yu, Y.; Deng, Y.; Bi, C.; Zhang, L.; Zhang, H. Assessing the dynamic extrusion-based 3D printing process for power-law fluid using numerical simulation. J. Food Eng. 2020, 275, 109861. [Google Scholar] [CrossRef]
- Maldonado-Rosas, R.; Tejada-Ortigoza, V.; Cuan-Urquizo, E.; Mendoza-Cachú, D.; la Peña, M.M.-D.; Alvarado-Orozco, J.M.; Campanella, O.H. Evaluation of rheology and printability of 3D printing nutritious food with complex formulations. Addit. Manuf. 2022, 58, 103030. [Google Scholar] [CrossRef]
- Nijdam, J.J.; LeCorre-Bordes, D.; Delvart, A.; Schon, B.S. A rheological test to assess the ability of food inks to form dimensionally stable 3D food structures. J. Food Eng. 2021, 291, 110235. [Google Scholar] [CrossRef]
- Páez, L.C.; Pirajan, I.D.; Urrea, M.C.; Sánchez, R.M.; Gómez, M.; Monroy, L.A. Comparison of cell culture HeLa and HEp-2: Studies prospects Chlamydia trachomatis. Nova 2015, 13, 17–29. [Google Scholar]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 1–15. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.A.; Gaudenzi, G.; Russom, A. 3D Bioprinting of Multi-Material Decellularized Liver Matrix Hydrogel at Physiological Temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.-J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rego, J.M.; Mendoza-Cerezo, L.; Macías-García, A.; Carrasco-Amador, J.P.; Marcos-Romero, A.C. Methodology for characterizing the printability of hydrogels. Int. J. Bioprinting 2023, 9, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Ching, T.; Hashimoto, M. Bioprinting using PEGDMA-based hydrogel on DLP printer. Mater. Today Proc. 2022, 70, 179–183. [Google Scholar] [CrossRef]
- Martyniak, K.; Lokshina, A.; Cruz, M.A.; Karimzadeh, M.; Kemp, R.; Kean, T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022, 152, 221–234. [Google Scholar] [CrossRef]
- Carpentier, N.; Van der Meeren, L.; Skirtach, A.G.; Devisscher, L.; Van Vlierberghe, H.; Dubruel, P.; Van Vlierberghe, S. Gelatin-Based Hybrid Hydrogel Scaffolds: Toward Physicochemical Liver Mimicry. Biomacromolecules 2022, 24, 4333–4347. [Google Scholar] [CrossRef]
- Bercea, M. Rheology as a Tool for Fine-Tuning the Properties of Printable Bioinspired Gels. Molecules 2023, 28, 2766. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Yang, J.; Yang, Y.; Ding, H.; Yu, B.; Ma, K.; Chen, M. Gelatin methacryloyl (GelMA) loaded with concentrated hypoxic pretreated adipose-derived mesenchymal stem cells(ADSCs) conditioned medium promotes wound healing and vascular regeneration in aged skin. Biomater. Res. 2023, 27, 1–17. [Google Scholar] [CrossRef]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef]
- Rastin, H.; Ormsby, R.T.; Atkins, G.J.; Losic, D. 3D Bioprinting of Methylcellulose/Gelatin-Methacryloyl (MC/GelMA) Bioink with High Shape Integrity. ACS Appl. Bio Mater. 2020, 3, 1815–1826. [Google Scholar] [CrossRef]
- Anand, R.; Amoli, M.S.; Huysecom, A.-S.; Amorim, P.A.; Agten, H.; Geris, L.; Bloemen, V. A tunable gelatin-hyaluronan dialdehyde/methacryloyl gelatin interpenetrating polymer network hydrogel for additive tissue manufacturing. Biomed. Mater. 2022, 17, 045027. [Google Scholar] [CrossRef]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales, Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, UK, 2000; Volume 6, Available online: https://books.google.com/books/about/A_Handbook_of_Elementary_Rheology.html?hl=es&id=zBYvAQAAIAAJ (accessed on 20 January 2024).
- Delugo, L.; Cabrera, C.A.; Provasi, P.F. Flujo viscoso: Diseño y construcción de un dispositivo para determinar el coeficiente de viscosidad dinámica del agua. Ext. Innov. Transf. Tecnol. 2021, 7, 108. [Google Scholar] [CrossRef]
- Jochems, C.E.A.; van der Valk, J.B.; Stafleu, F.R.; Baumans, V. The Use of Fetal Bovine Serum: Ethical or Scientific Problem? Altern. Lab. Anim. 2002, 30, 219–227. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Aguilera, P.; Dubarry, M.; Geli, V.; Simon, M.-N. NPCs and APBs: Two HUBs of non-canonical homology-based recombination at telomeres? Cell Cycle 2023, 22, 1163–1168. [Google Scholar] [CrossRef]
- Tseng, A.; Inuzuka, H.; Gao, D.; Singh, A.; Wei, W. Chapter 12 Experimental Approaches to Investigate the Proteasomal Degradation Pathways Involved in Regulation of Apoptosis. Methods Enzym. 2008, 446, 205–223. [Google Scholar] [CrossRef]
- Choi, E.; Kim, D.; Kang, D.; Yang, G.H.; Jung, B.; Yeo, M.; Park, M.-J.; An, S.; Lee, K.; Kim, J.S.; et al. 3D-printed gelatin methacrylate (GelMA)/silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen. Biomater. 2021, 8, rbab001. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Priya, V.N.K.; Kim, J.-H.; Khatun, M.R.; Nagarajan, R.; Noh, I. Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate. Biomater. Res. 2022, 26, 1–12. [Google Scholar] [CrossRef]
- Heidenreich, A.C.; Pérez-Recalde, M.; GonzálezWusener, A.; Hermida, É.B. Collagen and chitosan blends for 3D bioprinting: A rheological and printability approach. Polym. Test. 2020, 82, 106297. [Google Scholar] [CrossRef]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of Alginate-Carboxymethyl Cellulose Hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef]
- Lin, J.; Chen, L.; Jiang, W.; Zhang, H.; Shi, Y.; Cai, W. Rapid detection of low-level HeLa cell contamination in cell culture using nested PCR. J. Cell. Mol. Med. 2019, 23, 227–236. [Google Scholar] [CrossRef]








| Concentration | Proportion | Hydrogel Nomenclature | |
|---|---|---|---|
| GelMA 10% | DMEM | ||
| 100% | 0% | 1:0 | GelMA 100% |
| 75% | 25% | 3:1 | GelMA 75% |
| 50% | 50% | 1:1 | GelMA 50% |
| Hydrogel | Printing Temperature (°C) | Pressure [kPa] | Injector Used | Impression Plate Temperature (°C) | Photoreticulation Module | Exposure Time to Ultraviolet Light (s) |
|---|---|---|---|---|---|---|
| Cellink Start | 30 | 81 | 22 G | 20 | None | None |
| GelMA 100% | 30 | 145 | 22 G | 20 | 365 nm | 45 |
| GelMA 75% | 30 | 138 | 22 G | 20 | 365 nm | 45 |
| GelMA 50% | 30 | 118 | 22 G | 20 | 365 nm | 45 |
| Real Value (mm2) | Theoretical Value (mm2) | Deviation (mm2) | |
|---|---|---|---|
| Row 1 | - | 4.00 | - |
| 4.29 | 8.00 | 3.71 | |
| 7.67 | 16.00 | 8.33 | |
| 11.52 | 32.00 | 20.48 | |
| Row 2 | 6.88 | 8.00 | 1.12 |
| 12.54 | 16.00 | 3.46 | |
| 25.50 | 32.00 | 6.50 | |
| 57.35 | 64.00 | 6.65 | |
| Row 3 | 6.30 | 12.00 | 5.70 |
| 19.19 | 24.00 | 4.81 | |
| 42.10 | 48.00 | 5.90 | |
| 92.24 | 96.00 | 3.76 | |
| Row 4 | 12.00 | 16.00 | 4.00 |
| 27.32 | 32.00 | 4.68 | |
| 56.70 | 64.00 | 7.30 | |
| 119.32 | 128.00 | 8.68 | |
| Row 5 | 11.70 | 20.00 | 8.30 |
| 32.67 | 40.00 | 7.33 | |
| 67.14 | 80.00 | 12.86 | |
| 154.00 | 160.00 | 6.00 |
| Real Value (mm2) | Theoretical Value (mm2) | Deviation (mm2) | |
|---|---|---|---|
| Row 1 | - | 4.00 | - |
| 4.20 | 8.00 | 3.80 | |
| 13.95 | 16.00 | 2.05 | |
| 14.50 | 32.00 | 17.50 | |
| Row 2 | 4.70 | 8.00 | 3.30 |
| 7.50 | 16.00 | 8.50 | |
| 25.78 | 32.00 | 6.22 | |
| 51.20 | 64.00 | 12.80 | |
| Row 3 | 6.10 | 12.00 | 5.90 |
| 19.92 | 24.00 | 4.08 | |
| 39.68 | 48.00 | 8.32 | |
| 87.78 | 96.00 | 8.22 | |
| Row 4 | 10.60 | 16.00 | 5.40 |
| 24.80 | 32.00 | 7.20 | |
| 52.10 | 64.00 | 11.90 | |
| 124.20 | 128.00 | 3.80 | |
| Row 5 | 12.00 | 20.00 | 8.00 |
| 25.12 | 40.00 | 14.88 | |
| 69.24 | 80.00 | 10.76 | |
| 145.64 | 160.00 | 14.36 |
| Number of Samples | Average Deviation Produced (mm2) | % Similarity to Theoretical Value | |
|---|---|---|---|
| Theoretical value x < 20 mm2 | 7.00 | 4.39 | 56.20 |
| Theoretical value 20 mm2 ≤ x < 64 mm2 | 7.00 | 8.29 | 72.70 |
| Theoretical value ≥ 64 mm2 | 6.00 | 7.54 | 91.28 |
| Number of Samples | Average Deviation Produced (mm2) | % Similarity to Theoretical Value | |
|---|---|---|---|
| Theoretical value x < 20 | 7.00 | 4.83 | 51.77 |
| Theoretical value 20 ≤ x < 64 | 7.00 | 9.46 | 70.26 |
| Theoretical value ≥ 64 | 6.00 | 10.31 | 87.91 |
| GelMA 75% | GelMA 50% | GelMA | |
|---|---|---|---|
| Theoretical value x < 20 | 56.20 | 51.77 | 76.76 |
| Theoretical value 20 ≤ x < 64 | 72.70 | 70.26 | 88.58 |
| Theoretical value ≥ 64 | 91.28 | 87.91 | 91.85 |
| Hydrogel | Cf1 | Cf2 | Cf3 | Cf4 | Cf5 | Cf6 | Cf7 | Cf8 |
|---|---|---|---|---|---|---|---|---|
| GelMA 100% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.85 | 0.95 | 1.10 |
| GelMA 75% | 0.00 | 0.00 | 1.52 | 2.23 | 3.23 | 6.48 | 8.48 | 8.20 |
| GelMA 50% | 0.00 | 0.00 | 2.27 | 3.03 | 7.86 | 10.67 | 14.70 | 15.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Cerezo, L.; Rodríguez-Rego, J.M.; Macías-García, A.; Callejas-Marín, A.; Sánchez-Guardado, L.; Marcos-Romero, A.C. Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration. Polymers 2024, 16, 1437. https://doi.org/10.3390/polym16101437
Mendoza-Cerezo L, Rodríguez-Rego JM, Macías-García A, Callejas-Marín A, Sánchez-Guardado L, Marcos-Romero AC. Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration. Polymers. 2024; 16(10):1437. https://doi.org/10.3390/polym16101437
Chicago/Turabian StyleMendoza-Cerezo, Laura, Jesús M. Rodríguez-Rego, Antonio Macías-García, Antuca Callejas-Marín, Luís Sánchez-Guardado, and Alfonso C. Marcos-Romero. 2024. "Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration" Polymers 16, no. 10: 1437. https://doi.org/10.3390/polym16101437










