Anti-Metastatic Effects of Standardized Polysaccharide Fraction from Diospyros kaki Leaves via GSK3β/β-Catenin and JNK Inactivation in Human Colon Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability
2.4. Wound-Healing Assay
2.5. Invasion Assay
2.6. qRT-PCR Assay
2.7. Western Blotting
2.8. Gelatin Zymography Assay
2.9. Transient Transfection and Luciferase Assay
2.10. Small-Interfering RNA (siRNA) Transfection
2.11. Statistical Analysis
3. Results
3.1. PLE0 Inhibits the Migration and Invasion of HT-29 and HCT-116
3.2. PLE0 Inhibits Mesenchymal and Epithelial Phenotypes in HT-29 Cells
3.3. The Activity and Expression of MMP-2 and MMP-9 by PLE0 in HT-29 Cells
3.4. PLE0 Suppresses the Activation of NF-κB and AP-1 in HT-29 Cells
3.5. PLE0 Inhibits Metastasis via JNK Inactivation in HT-29 Cells
4. Discussion
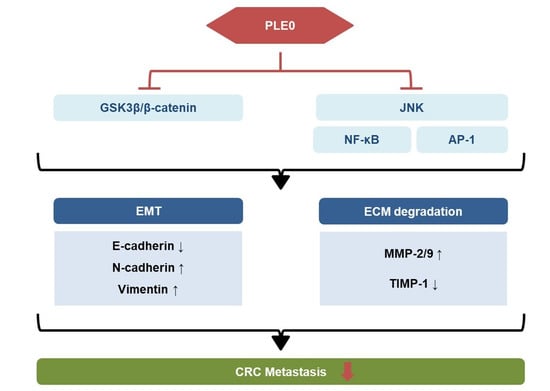
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal. Transduct. Target. Ther. 2022, 7, 70. [Google Scholar] [CrossRef]
- Andriole, V.T. Economic problems facing the infectious disease academic division—The perspective of a division chief. Bull. N. Y. Acad. Med. 1988, 64, 557–564. [Google Scholar]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef]
- Son, J.E.; Hwang, M.K.; Lee, E.; Seo, S.G.; Kim, J.E.; Jung, S.K.; Kim, J.R.; Ahn, G.H.; Lee, K.W.; Lee, H.J. Persimmon peel extract attenuates PDGF-BB-induced human aortic smooth muscle cell migration and invasion through inhibition of c-Src activity. Food Chem. 2013, 141, 3309–3316. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Huh, G.; Jung, S.H.; Kwon, H.; Jeon, Y.; Park, Y.N.; Kim, Y.J. Diospyros kaki leaves inhibit HGF/Met signaling-mediated EMT and stemness features in hepatocellular carcinoma. Food Chem. Toxicol. 2020, 142, 111475. [Google Scholar] [CrossRef]
- Albersheim, P.; An, J.; Freshour, G.; Fuller, M.S.; Guillen, R.; Ham, K.S.; Hahn, M.G.; Huang, J.; O’Neill, M.; Whitcombe, A.; et al. Structure and function studies of plant cell wall polysaccharides. Biochem. Soc. Trans. 1994, 22, 374–378. [Google Scholar] [CrossRef]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Hong, H.D. Immunostimulatory effects of polysaccharides isolated from Makgeolli (traditional Korean rice wine). Molecules 2014, 19, 5266–5277. [Google Scholar] [CrossRef]
- Perez, S.; Rodriguez-Carvajal, M.A.; Doco, T. A complex plant cell wall polysaccharide: Rhamnogalacturonan II. A structure in quest of a function. Biochimie 2003, 85, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hilz, H.; Williams, P.; Doco, T.; Schols, H.A.; Voragen, A.G.J. The pectic polysaccharide rhamnogalacturonan II is present as a dimer in pectic populations of bilberries and black currants in muro and in juice. Carbohydr. Polym. 2006, 65, 521–528. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, H.R.; Shin, M.S.; Cho, S.Y.; Choi, H.J.; Shin, K.S. Antitumor metastasis activity of pectic polysaccharide purified from the peels of Korean Citrus Hallabong. Carbohydr. Polym. 2014, 111, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Hong, H.D.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shin, M.S. Inhibitory Effects of Pectic Polysaccharide Isolated from Diospyros kaki Leaves on Tumor Cell Angiogenesis via VEGF and MMP-9 Regulation. Polymers 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Choi, J.W.; Song, N.E.; Cho, C.W.; Rhee, Y.K.; Hong, H.D. Polysaccharide isolated from persimmon leaves (Diospyros kaki Thunb.) suppresses TGF-beta1-induced epithelial-to-mesenchymal transition in A549 cells. Int. J. Biol. Macromol. 2020, 164, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Chung, K.S.; Heo, S.W.; Kim, S.Y.; Lee, J.H.; Hassan, A.H.E.; Lee, Y.S.; Lee, J.Y.; Lee, K.T. Therapeutic role of 2-stearoxyphenethyl phosphocholine targeting microtubule dynamics and Wnt/beta-catenin/EMT signaling in human colorectal cancer cells. Life Sci. 2023, 334, 122227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Shin, J.S.; Chung, K.S.; Kim, J.M.; Jung, S.H.; Yoo, H.S.; Hassan, A.H.E.; Lee, J.K.; Inn, K.S.; Lee, S.; et al. 3′,4′-Dihydroxyflavone mitigates inflammatory responses by inhibiting LPS and TLR4/MD2 interaction. Phytomedicine 2023, 109, 154553. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Chung, K.S.; Jang, S.Y.; Han, H.S.; Heo, S.W.; Lee, J.K.; Kim, H.J.; Shin, Y.K.; Ahn, H.S.; Lee, S.H.; et al. Hydrangenol, an active constituent of Hydrangea serrata (Thunb.) Ser., ameliorates colitis through suppression of macrophage-mediated inflammation in dextran sulfate sodium-treated mice. Food Funct. 2023, 14, 6957–6968. [Google Scholar] [CrossRef]
- Cecen, B.; Keles, D.; Oktay, G.; Kozaci, L.D. Effects of simvastatin on matrix metalloproteinase regulation in IL-1beta-induced SW1353 cells. Chem. Biol. Interact. 2019, 310, 108730. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, Y.K.; Lee, W.S.; Park, O.J.; Kim, Y.M. The involvement of AMPK/GSK3-beta signals in the control of metastasis and proliferation in hepato-carcinoma cells treated with anthocyanins extracted from Korea wild berry Meoru. BMC Complement. Altern. Med. 2014, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Pietruszewska, W.; Bojanowska-Pozniak, K.; Kobos, J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An immunohistochemical study. Pol. J. Otolaryngol. 2016, 70, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117 Pt 25, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-kappaB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecinska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ung, T.T.; Nguyen, T.T.; Sah, D.K.; Park, S.Y.; Jung, Y.D. Cholic Acid Stimulates MMP-9 in Human Colon Cancer Cells via Activation of MAPK, AP-1, and NF-kappaB Activity. Int. J. Mol. Sci. 2020, 21, 3420. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Khraiwesh, H.; Abdelrahim, D.N.; Mahmoud, I.F.; Faris, M. Knowledge, Awareness, and Practices toward Colorectal Cancer and Its Dietary and Lifestyle-Related Risk Factors among Jordanian University Students: A Cross-Sectional Study. J. Cancer Epidemiol. 2024, 2024, 4503448. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, P.; Zhang, Q.; Zhao, S.; Dang, Z.; Lu, M.; Li, H.; Zhang, Y.; Wang, T. Colorectal cancer inhibitory properties of polysaccharides and their molecular mechanisms: A review. Int. J. Biol. Macromol. 2023, 238, 124165. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, J.; Jiang, J.; Li, Y.; Gan, Z.; Ding, Y.; Li, Y.; Liu, J.; Wang, S.; Ke, Y. Characterization of polysaccharide from Scutellaria barbata and its antagonistic effect on the migration and invasion of HT-29 colorectal cancer cells induced by TGF-beta1. Int. J. Biol. Macromol. 2019, 131, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.Q.; Sheng, X.; Qin, J.M.; Sun, K.; Zhao, W.; Ni, L. The effect and mechanism of bufalin on regulating hepatocellular carcinoma cell invasion and metastasis via Wnt/beta-catenin signaling pathway. Int. J. Oncol. 2016, 48, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.Y.; Lin, C.Y.; Yu, C.C.; Chen, H.T.; Lien, M.Y.; Huang, Y.W.; Fong, Y.C.; Liu, J.F.; Wang, S.W.; Chen, W.C.; et al. Visfatin Promotes the Metastatic Potential of Chondrosarcoma Cells by Stimulating AP-1-Dependent MMP-2 Production in the MAPK Pathway. Int. J. Mol. Sci. 2021, 22, 8642. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, M.; Abassi, Y.; Kifagi, C.; Fezai, M.; Daams, R.; Lang, F.; Massoumi, R. Reversine inhibits Colon Carcinoma Cell Migration by Targeting JNK1. Sci. Rep. 2018, 8, 11821. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, T.; Tang, Y.; Li, Q.; Wang, J.; Cheng, X.; Zhang, W.; Zhang, B.; Zhou, C.; Tu, S. Utidelone inhibits growth of colorectal cancer cells through ROS/JNK signaling pathway. Cell Death Dis. 2021, 12, 338. [Google Scholar] [CrossRef]
- Tam, S.Y.; Law, H.K. JNK in Tumor Microenvironment: Present Findings and Challenges in Clinical Translation. Cancers 2021, 13, 2196. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-S.; Shin, J.-S.; Jang, S.-Y.; Chung, K.-S.; Kim, S.-D.; Cho, C.-W.; Hong, H.-D.; Rhee, Y.K.; Lee, K.-T. Anti-Metastatic Effects of Standardized Polysaccharide Fraction from Diospyros kaki Leaves via GSK3β/β-Catenin and JNK Inactivation in Human Colon Cancer Cells. Polymers 2024, 16, 1275. https://doi.org/10.3390/polym16091275
Lee W-S, Shin J-S, Jang S-Y, Chung K-S, Kim S-D, Cho C-W, Hong H-D, Rhee YK, Lee K-T. Anti-Metastatic Effects of Standardized Polysaccharide Fraction from Diospyros kaki Leaves via GSK3β/β-Catenin and JNK Inactivation in Human Colon Cancer Cells. Polymers. 2024; 16(9):1275. https://doi.org/10.3390/polym16091275
Chicago/Turabian StyleLee, Woo-Seok, Ji-Sun Shin, Seo-Yun Jang, Kyung-Sook Chung, Soo-Dong Kim, Chang-Won Cho, Hee-Do Hong, Young Kyoung Rhee, and Kyung-Tae Lee. 2024. "Anti-Metastatic Effects of Standardized Polysaccharide Fraction from Diospyros kaki Leaves via GSK3β/β-Catenin and JNK Inactivation in Human Colon Cancer Cells" Polymers 16, no. 9: 1275. https://doi.org/10.3390/polym16091275