The Physiological Adjustments of Two Xerophytic Shrubs to Long-Term Summer Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Gas-Exchange Measurements
2.3. Specific Leaf Area and N Concentration
2.4. Hydrometeorological Measurements
2.5. Data Analysis
3. Results
3.1. Seasonal Changes in Environmental Factors
3.2. Seasonal Changes in Asat, gs, and E
3.3. Seasonal Changes in LMA and Narea
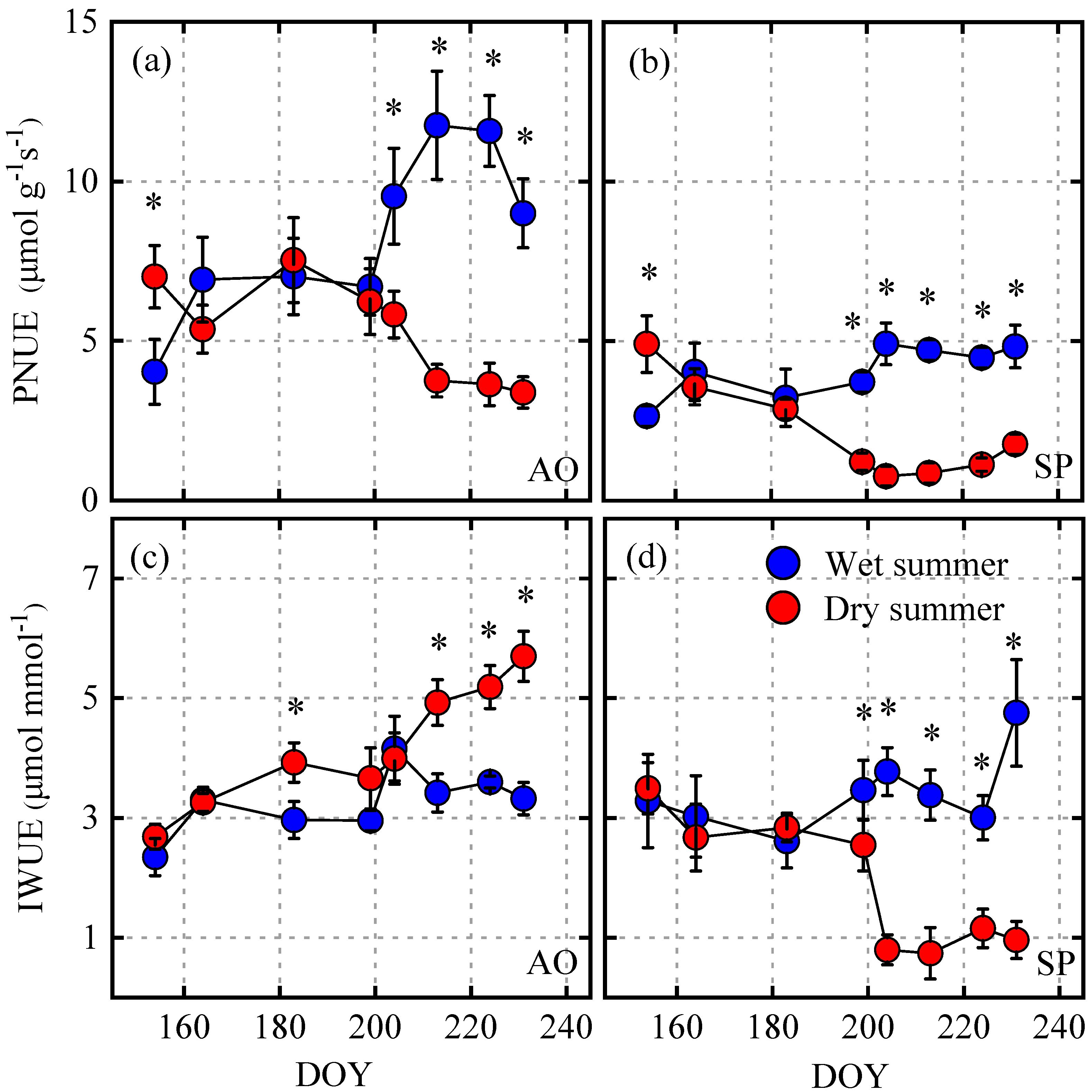
3.4. Seasonal Changes in the PNUE and IWUE and Their Controlling Factors
4. Discussion
4.1. Seasonal Response of Leaf Photosynthesis to Long-Term Summer Drought
4.2. Tradeoffs between the IWUE and PNUE during Long-Term Summer Drought
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, S.; Chen, L.; Shankman, D.; Wang, C.; Wang, X.; Zhang, H. Excessive reliance on afforestation in China’s arid and semi-arid regions: Lessons in ecological restoration. Earth Sci. Rev. 2011, 104, 240–245. [Google Scholar] [CrossRef]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villa, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Carvajal, D.E.; Loayza, A.P.; Rios, R.S.; Delpiano, C.A.; Squeo, F.A. A hyper-arid environment shapes an inverse pattern of the fast–slow plant economics spectrum for above-, but not below-ground resource acquisition strategies. J. Ecol. 2019, 107, 1079–1092. [Google Scholar] [CrossRef]
- Wang, Y.P.; Chen, Q.; Zheng, J.Z.; Zhang, Z.Z.; Gao, T.T.; Li, C.; Ma, F.W. Overexpression of the tyrosine decarboxylase gene MdTyDC in apple enhances long-term moderate drought tolerance and WUE. Plant Sci. 2021, 313, 111064. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q.; Liu, B.; Davis, M.; Sardans, J.; Peñuelas, J.; Billings, S. Long-term nitrogen deposition linked to reduced water use efficiency in forests with low phosphorus availability. New Phytol. 2016, 210, 431–442. [Google Scholar] [CrossRef]
- Liu, P.; Black, T.A.; Jassal, R.S.; Zha, T.S.; Nesic, Z.; Barr, A.G.; Helgason, W.D.; Jia, X.; Tian, Y.; Stephens, J.J.; et al. Divergent long-term trends and interannual variation in ecosystem resource use efficiencies of a southern boreal old black spruce forest 1999–2017. Glob. Chang. Biol. 2019, 25, 3056–3069. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, J.F.; Zhang, Z.Q.; Ollinger, S.V.; Hollinger, D.Y.; Pan, Y.; Wan, J.M. Canopy photosynthetic capacity drives contrasting age dynamics of resource use efficiencies between mature temperate evergreen and deciduous forests. Glob. Chang. Biol. 2020, 26, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Osuna, L.J.; Baldocchi, D.D.; Kobayashi, H.; Dawson, T.E. Seasonal trends in photosynthesis and electron transport during the Mediterranean summer drought in leaves of deciduous oaks. Tree Physiol. 2015, 35, 485–500. [Google Scholar] [CrossRef]
- Liu, D.; Llusia, J.; Ogaya, R.; Estiarte, M.; Llorens, L.; Yang, X.H.; Peñuelas, J. Physiological adjustments of a Mediterranean shrub to long-term experimental warming and drought treatments. Plant Sci. 2016, 252, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zha, T.S.; Bourque, C.P.A.; Liu, P.; Jia, J.; Zhang, F.; Yu, H.Q.; Tian, Y.; Li, X.H.; Kang, X.Y.; et al. Multi-year trends and interannual variation in ecosystem resource use efficiencies in a young mixed wood plantation in northern China. Agric. For. Meteorol. 2023, 330, 109318. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Volaire, F.; Fattet, M.; Blanchard, A.; Roumet, C. Tradeoffs between functional strategies for resource-use and drought-survival in Mediterranean rangeland species. Environ. Exp. Bot. 2013, 87, 126–136. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, Y.; Zha, T.S.; Jia, X.; Bourque, C.P.A.; Liu, P.; Jin, C.; Jiang, X.Y.; Li, X.H.; Wei, N.N.; et al. Dynamic changes in plant resource use efficiencies and their primary influence mechanisms in a typical desert shrub community. Forests 2021, 12, 1372. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Maseyk, K.; Hemming, D.; Angert, A.; Leavitt, S.W.; Yakir, D. Increase in water-use efficiency and underlying processes in pine forests. across a precipitation gradient in the dry Mediterranean region over the past 30 years. Oecologia 2011, 167, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Hai, X.Y.; Li, J.P.; Li, J.W.; Liu, Y.X.; Dong, L.B.; Wang, X.Z.; Lv, W.W.; Hu, Z.H.; Shangguan, Z.P.; Deng, L. Variations in plant water use efficiency response to manipulated precipitation in a temperate grassland. Front. Plant Sci. 2022, 13, 881282. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.J.; Zha, T.S.; Bourque, C.P.A.; Jia, X.; Ma, J.Y.; Liu, P.; Yang, R.Z.; Li, C.; Du, T.; Wu, Y.J. Variation in ecosystem water use efficiency along a southwest-to-northeast aridity gradient in China. Ecol. Indic. 2020, 110, 105932. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Gou, X.H.; Fonti, P.; Xia, J.Q.; Cao, Z.Y.; Liu, J.G.; Wang, Y.F.; Zhang, J.Z. Seasonal variations in leaf-level photosynthesis and water use efficiency of three isohydric to anisohydric conifers on the Tibetan Plateau. Agric. For. Meteorol. 2021, 308–309, 108581. [Google Scholar] [CrossRef]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valiente-Banuet, A.; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global leaf trait relationships: Mass, area, and the leaf economics spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Guo, R.Q.; Sun, S.C.; Liu, B. Difference in leaf water use efficiency/photosynthetic nitrogen use efficiency of Bt-cotton and its conventional peer. Sci. Rep. 2016, 6, 33539. [Google Scholar] [CrossRef]
- Limousin, J.M.; Yepez, E.A.; McDowell, N.G.; Pockman, W.T. Convergence in resource use efficiency across trees with differing hydraulic strategies in response to ecosystem precipitation manipulation. Funct. Ecol. 2015, 29, 1125–1136. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Tateno, R.; Taniguchi, T.; Zhang, J.; Shi, W.Y.; Zhang, J.G.; Du, S.; Yamanaka, N. Net primary production, nitrogen cycling, biomass allocation, and resource use efficiency along a topographical soil water and nitrogen gradient in a semi-arid forest near an arid boundary. Plant Soil 2017, 420, 209–222. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants—An economic analogy. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Patterson, T.B.; Guy, R.D.; Dang, Q.L. Whole-plant nitrogen- and water-relations traits, and their associated trade-offs, in adjacent muskeg and upland boreal spruce species. Oecologia 1997, 110, 160–168. [Google Scholar] [CrossRef]
- Hirose, T.; Bazzaz, F.A. Trade-off between light- and nitrogen-use efficiency in canopy photosynthesis. Ann. Bot. 1998, 82, 195–202. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about efficiency of resource use in forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Gong, X.Y.; Chen, Q.; Lin, S.; Brueck, H.; Dittert, K.; Taube, F.; Schnyder, H. Tradeoffs between nitrogen- and water-use efficiency in dominant species of the semiarid steppe of Inner Mongolia. Plant Soil 2011, 340, 227–238. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Carrillo, Y.; Aspinwall, M.J.; Maier, C.; Canarini, A.; Tahaei, H.; Choat, B.; Tissue, D.T. Water, nitrogen and phosphorus use efficiencies of four tree species in response to variable water and nutrient supply. Plant Soil 2016, 406, 187–199. [Google Scholar] [CrossRef]
- Tarvainen, L.; Räntfors, M.; Wallin, G. Seasonal and within-canopy variation in shoot-scale resource-use efficiency trade-offs in a Norway spruce stand. Plant Cell Environ. 2015, 38, 2487–2496. [Google Scholar] [CrossRef]
- Xu, L.K.; Baldocchi, D.D. Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 2003, 23, 865–877. [Google Scholar] [CrossRef]
- Miller, J.M.; Williams, R.J.; Farquhar, G.D. Carbon isotope discrimination by a sequence of Eucalyptus species along a subcontinental rainfall gradient in Australia. Funct. Ecol. 2001, 15, 222–232. [Google Scholar] [CrossRef]
- Santos, V.A.H.F.d.; Ferreira, M.J.; Rodrigues, J.V.F.C.R.; Garcia, M.N.; Ceron, J.V.B.; Nelson, B.W.; Saleska, S.R. Causes of reduced leaf-level photosynthesis during strong El Nino drought in a central Amazon Forest. Glob. Chang. Biol. 2018, 24, 4266–4279. [Google Scholar] [CrossRef]
- Gyenge, J.; Fernández, M.E. Short- and long-term responses to seasonal drought in ponderosa pines growing at different plantation densities in Patagonia, South America. Trees 2012, 26, 1905–1917. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.S.; Gong, J.N.; Zhang, Y.Q.; Wu, B.; Qin, S.G. Multi-scale dynamics and environmental controls on net ecosystem CO2 exchange over a temperate semiarid shrubland. Agric. For. Meteorol. 2018, 259, 250–259. [Google Scholar] [CrossRef]
- Iqbal, S.; Zha, T.S.; Jia, X.; Hayat, M.; Qian, D.; Bourque, C.P.A.; Tian, Y.; Liu, P.; Yang, R.Z.; Khan, A. Interannual variation in sap flow response in three xeric shrub species to periodic drought. Agric. For. Meteorol. 2021, 297, 108276. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhang, Y.Q.; Feng, W.; Qin, S.G.; Liu, Z. Revegetated shrub species recruit different soil fungal assemblages in a desert ecosystem. Plant Soil 2019, 435, 81–93. [Google Scholar] [CrossRef]
- Xiao, C.W.; Zhou, G.S.; Zhang, X.S.; Zhao, J.Z.; Wu, G. Responses of dominant desert species Artemisia ordosica and Salix psammophila to water stress. Photosynthetica 2005, 43, 467–471. [Google Scholar] [CrossRef]
- Yang, H.B.; An, S.Q.; Sun, J.X.; Shi, Z.M.; She, X.S.; Sun, Q.Y.; Liu, S.R. Seasonal Variation and correlation with environmental factors of photosynthesis and water use efficiency of Juglans regia and Ziziphus jujuba. J. Integr. Plant Biol. 2008, 50, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.R.; Zhang, Y.Q.; Liu, J.B.; Wu, B.; Qin, S.G.; Fa, K.Y. Fine-root distribution, production, decomposition, and effect on soil organic carbon of three revegetation shrub species in northwest China. For. Ecol. Manag. 2016, 359, 381–388. [Google Scholar] [CrossRef]
- Han, Y.N.; Wu, J.Y.; Tian, Y.; Zha, T.S.; Jia, X.; Bourque, C.P.A.; Wu, Y.J.; Bai, Y.J.; Ma, J.Y.; Zhang, M.Y. Light energy partitioning and photoprotection in an exotic species (Salix psammophila) grown in a semi-arid area of northwestern China. Forests 2018, 9, 341. [Google Scholar] [CrossRef]
- Wu, Y.J.; Ren, C.; Tian, Y.; Zha, T.S.; Liu, P.; Bai, Y.J.; Ma, J.Y.; Lai, Z.R.; Bourque, C.P.A. Photosynthetic gas-exchange and PSII photochemical acclimation to drought in a native and non-native xerophytic species (Artemisia ordosica and Salix psammophila). Ecol. Indic. 2018, 94, 130–138. [Google Scholar] [CrossRef]
- Xu, M.Z.; Zha, T.S.; Tian, Y.; Liu, P.; Jia, X.; Bourque, C.P.A.; Jin, C.; Wei, X.S.; Zhao, H.X.; Guo, Z.F. Elevated physiological plasticity in xerophytic-deciduous shrubs as demonstrated in their variable maximum carboxylation rate. Ecol. Indic. 2022, 144, 109475. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.S.; Gong, J.N.; Wang, B.; Zhang, Y.Q.; Wu, B.; Qin, S.G.; Peltola, H. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 2016, 228, 120–129. [Google Scholar]
- Jiang, X.Y.; Jia, X.; Gao, S.J.; Jiang, Y.; Wei, N.N.; Han, C.; Zha, T.S.; Liu, P.; Tian, Y.; Qin, S.G. Plant nutrient contents rather than physical traits are coordinated between leaves and roots in a desert shrubland. Front. Plant Sci. 2021, 12, 734775. [Google Scholar] [CrossRef]
- Xie, J.; Zha, T.S.; Jia, X.; Qian, D.; Wu, B.; Zhang, Y.Q.; Bourque, C.P.A.; Chen, J.Q.; Sun, G.; Peltola, H. Irregular precipitation events in control of seasonal variations in CO2 exchange in a cold desert-shrub ecosystem in northwest China. J. Arid Environ. 2015, 120, 33–41. [Google Scholar] [CrossRef]
- Zha, T.S.; Qian, D.; Jia, X.; Bai, Y.J.; Tian, Y.; Bourque, C.P.A.; Ma, J.Y.; Feng, W.; Wu, B.; Peltola, H. Soil moisture control of sap-flow response to biophysical factors in a desert-shrub species, Artemisia ordosica. Biogeosciences 2017, 14, 4533–4544. [Google Scholar] [CrossRef]
- Harrison, S.; LaForgia, M. Seedling traits predict drought-induced mortality linked to diversity loss. Proc. Natl. Acad. Sci. USA 2019, 116, 12. [Google Scholar] [CrossRef]
- Hoshika, Y.; Paoletti, E.; Centritto, M.; Gomes, M.T.G.; Puértolas, J.; Haworth, M. Species-specific variation of photosynthesis and mesophyll conductance to ozone and drought in three Mediterranean oaks. Physiol. Plant. 2022, 174, e13639. [Google Scholar] [CrossRef]
- Posch, S.; Bennett, L.T. Photosynthesis, photochemistry and antioxidative defence in response to two drought severities and with re-watering in Allocasuarina luehmannii. Plant Biol. 2009, 11, 83–93. [Google Scholar] [CrossRef]
- Durand, M.; Brendel, O.; Bure, C.; Thiec, D.L. Altered stomatal dynamics induced by changes in irradiance and vapour-pressure deficit under drought: Impacts on the whole-plant transpiration efficiency of poplar genotypes. New Phytol. 2019, 222, 1789–1802. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, Y.G.; Guo, K.; Li, G.Q.; Zheng, Y.R.; Yu, L.F.; Yang, R. Comparative ecophysiological responses to drought of two shrub and four tree species from karst habitats of southwestern China. Trees 2011, 25, 537–549. [Google Scholar] [CrossRef]
- Ávila-Lovera, E.; Urich, R.; Coronel, I.; Tezara, W. Seasonal gas exchange and resource-use efficiency in evergreen versus deciduous species from a tropical dry forest. Tree Physiol. 2019, 39, 1561–1571. [Google Scholar] [CrossRef]
- Tezara, W.; Marín, O.; Rengifo, E.; Martínez, D.; Herrera, A. Photosynthesis and photoinhibition in two xerophytic shrubs during drought. Photosynthetica 2005, 43, 37–45. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef]
- Ens, E.; Hutley, L.B.; Rossiter-Rachor, N.A.; Douglas, M.M.; Setterfield, S.A. Resource-use efficiency explains grassy weed invasion in a low resource savanna in north Australia. Front. Plant Sci. 2015, 6, 560. [Google Scholar] [CrossRef]
- Dalmolin, C.A.; Lobo, F.d.A.; Vourlitis, G.L.; Dalmagro, H.J.; Junior, M.Z.A.; Orti, C.E.R. Physiological adjustments of an invasive tree species to extreme hydrological events in a tropical seasonal wetland. Trees 2018, 32, 1365–1375. [Google Scholar] [CrossRef]
- Bartholomew, D.C.; Bittencourt, P.R.L.; da Costa, A.C.L.; Banin, L.F.; Costa, P.D.B.; Coughlin, S.I.; Domingues, T.F.; Ferreira, L.V.; Giles, A.; Mencuccini, M.; et al. Small tropical forest trees have a greater capacity to adjust carbon metabolism to long-term drought than large canopy trees. Plant Cell Environ. 2020, 43, 2380–2393. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M.; Prieto, P. Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Glob. Chang. Biol. 2008, 14, 2304–2316. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Hu, Y.Y.; Luo, H.H.; Chow, W.S.; Zhang, W.F. Two distinct strategies of cotton and soybean differing in leaf movement to perform photosynthesis under drought in the field. Funct. Plant Biol. 2011, 38, 567–575. [Google Scholar] [CrossRef]
- Eamus, D.; Myers, B.; Duff, G.; Williams, D. Seasonal changes in photosynthesis of eight savanna tree species. Tree Physiol. 1999, 19, 665–671. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C. Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 2003, 134, 455–462. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia: Photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Donovan, L.A.; Dudley, S.A.; Rosenthal, D.M.; Ludwig, F. Phenotypic selection on leaf water use effciency and related ecophysiological traits for natural populations of desert sunfowers. Oecologia 2007, 152, 13–25. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhou, G.S.; Zhou, L.; Lv, X.M.; Ji, Y.H.; Zho, M.Z. The interrelationship between water use efficiency and radiation use efficiency under progressive soil drying in Maize. Front. Plant Sci. 2021, 12, 794409. [Google Scholar] [CrossRef]
- Lázaro-Nogal, A.; Forner, A.; Traveset, A.; Valladares, F. Contrasting water strategies of two Mediterranean shrubs of limited distribution: Uncertain future under a drier climate. Tree Physiol. 2013, 33, 1284–1295. [Google Scholar] [CrossRef]
- Gorai, M.; Laajili, W.; Santiago, L.S.; Neffati, M. Rapid recovery of photosynthesis and water relations following soil drying and re-watering is related to the adaptation of desert shrub Ephedra alata subsp. Alenda (Ephedraceae) to arid environments. Environ. Exp. Bot. 2015, 109, 113–121. [Google Scholar] [CrossRef]
- Hu, H.Y.; Zhu, L.; Li, H.X.; Xu, D.M.; Xie, Y.Z. Seasonal changes in the water-use strategies of three herbaceous species in a native desert steppe of Ningxia, China. J. Arid. Land 2021, 13, 109–122. [Google Scholar] [CrossRef]
- Latif, A.A.; Olivier, N.; Stephane, M.O.; Langlade, N.B.; Thierry, L.; Philippe, G. Genetic control of water use efficiency and leaf carbon isotope discrimination in Sunflower (Helianthus annuus L.) subjected to two drought scenarios. PLoS ONE 2014, 9, e101218. [Google Scholar]
- Wang, Y.; Wang, Y.Z.; Tong, Y.H.; Zhu, X.G. Stomata conductance as a goalkeeper for increased photosynthetic efficiency. Curr. Opin. Plant Biol. 2022, 70, 102310. [Google Scholar] [CrossRef]
- Taylor, S.H.; Ripley, B.S.; Martin, T.; De-Wet, L.A.; Woodward, F.A.; Osborne, C.P. Physiological advantages of C4 grasses in the field: A comparative experiment demonstrating the importance of drought. Glob. Chang. Biol. 2014, 20, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Mereu, S.; Cherubini, P.; Castaldi, S.; Sirignano, C.; Lubritto, C.; Battipaglia, G. Tree-ring carbon and oxygen isotopes indicate different water use strategies in three Mediterranean shrubs at Capo Caccia (Sardinia, Italy). Trees 2015, 29, 1593–1603. [Google Scholar] [CrossRef]







| Variables | Species | |
|---|---|---|
| A. ordosica | S. psammophila | |
| Crown diameter (cm × cm) | 115 × 105 | 127 × 112 |
| Maximum height (cm) | 57 (5) | 222 (13) |
| Canopy coverage (%) | 82 (5) | 93 (3) |
| Aboveground biomass (g m−2) | 433 (32) | 2910 (78) |
| Variables | Season | Species | Season × Species | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Asat | 17.56 | <0.0001 | 38.15 | <0.0001 | 2.17 | 0.15 |
| gs | 42.17 | <0.0001 | 57.17 | <0.0001 | 10.62 | <0.01 |
| E | 17.61 | <0.001 | 34.16 | <0.001 | 7.81 | <0.05 |
| IWUE | 0.98 | 0.33 | 12.11 | <0.01 | 15.89 | <0.0001 |
| PNUE | 15.40 | <0.001 | 35.59 | <0.0001 | 0.69 | 0.41 |
| LMA | 2.03 | 0.09 | 22.73 | <0.0001 | 0.53 | 0.47 |
| Narea | 14.51 | <0.001 | 20.09 | <0.0001 | 0.21 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zha, T.; Tian, Y.; Liu, P.; Bourque, C.P.-A.; Jia, X.; Li, C.; Jin, C.; Guo, Z.; Wei, X. The Physiological Adjustments of Two Xerophytic Shrubs to Long-Term Summer Drought. Agronomy 2024, 14, 975. https://doi.org/10.3390/agronomy14050975
Xu M, Zha T, Tian Y, Liu P, Bourque CP-A, Jia X, Li C, Jin C, Guo Z, Wei X. The Physiological Adjustments of Two Xerophytic Shrubs to Long-Term Summer Drought. Agronomy. 2024; 14(5):975. https://doi.org/10.3390/agronomy14050975
Chicago/Turabian StyleXu, Mingze, Tianshan Zha, Yun Tian, Peng Liu, Charles P.-A. Bourque, Xin Jia, Cheng Li, Chuan Jin, Zifan Guo, and Xiaoshuai Wei. 2024. "The Physiological Adjustments of Two Xerophytic Shrubs to Long-Term Summer Drought" Agronomy 14, no. 5: 975. https://doi.org/10.3390/agronomy14050975





