Are Clay Minerals Systematically the Products of Aqueous Alteration in Cosmic Bodies?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Terrestrial Alteration Processes
2.2. Non-Altered Clay Mineral Textures
2.3. Aqueous Alteration in Meteorites
2.4. Alternative Interpretations of Chondrule Rim Petrographical Features
3. Conclusions
3.1. Nucleation and Propagation of Melting
3.2. Diffusion of Heat and Volatile Components from the Chondrule to the Matrix
3.3. Final Crystallization Stage and Hydrothermal Auto-Alteration
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grady, M.; Pratesi, G.; Moggi Cecchi, V. Atlas of Meteorites; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry, 2nd ed.; Holland, D.H., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 1–63. ISBN 9780080959757. [Google Scholar]
- Wiik, H. The chemical composition of some stony meteorites. Geochim. Cosmochim. Acta 1956, 9, 279–289. [Google Scholar] [CrossRef]
- Braukmüller, N.; Wombacher, F.; Hezel, D.C.; Escoube, R.; Münker, C. The chemical composition of carbonaceous chondrites: Implications for volatile element depletion, complementarity and alteration. Geochim. Cosmochim. Acta 2018, 239, 17–48. [Google Scholar] [CrossRef]
- Weisberg, M.K.; McCoy, T.J.; Krot, A.N. Systematics and evaluation of meteorite classification. In Meteorites and the early Solar System II; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006. [Google Scholar]
- Anders, E.; Ebihara, M. Solar-system abundances of the elements. Geochim. Cosmochim. Acta 1982, 46, 2363–2380. [Google Scholar] [CrossRef]
- Anders, E.; Grevesse, N. Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta 1989, 53, 197–214. [Google Scholar] [CrossRef]
- Barber, D.J. Phyllosilicates and other layer-structured materials in stony meteorites. Clay Miner. 1985, 20, 415–454. [Google Scholar] [CrossRef]
- Brearley, A.J. The Action of Water. Meteor. Early Sol. Syst. II 2006, 943, 587–624. [Google Scholar]
- Buseck, P.R.; Hua, X. Matrices of Carbonaceous Chondrite Meteorites. Annu. Rev. Earth Planet. Sci. 1993, 21, 255–305. [Google Scholar] [CrossRef]
- Nuth III, J.A.; Brearley, A.J.; Scott, E.R. Microcrystals and Amorphous Material in Comets and Primitive Meteorites: Keys to Understanding Processes in the Early Solar System. Proc. Chondrites Protoplanetary Disk 2005, 341, 675. [Google Scholar]
- Beck, P.; Quirico, E.; Montes-Hernandez, G.; Bonal, L.; Bollard, J.; Orthous-Daunay, F.-R.; Howard, K.; Schmitt, B.; Brissaud, O.; Deschamps, F.; et al. Hydrous mineralogy of CM and CI chondrites from infrared spectroscopy and their relationship with low albedo asteroids. Geochim. Cosmochim. Acta 2010, 74, 4881–4892. [Google Scholar] [CrossRef]
- Scott, E.R. Chondrites and the Protoplanetary Disk. Annu. Rev. Earth Planet. Sci. 2007, 35, 577–620. [Google Scholar] [CrossRef]
- Kleine, T.; Rudge, J.F. Chronometry of Meteorites and the Formation of the Earth and Moon. Elements 2011, 7, 41–46. [Google Scholar] [CrossRef]
- Trigo-Rodríguez, J.M.; Rimola, A.; Tanbakouei, S.; Soto, V.C.; Lee, M. Accretion of Water in Carbonaceous Chondrites: Current Evidence and Implications for the Delivery of Water to Early Earth. Space Sci. Rev. 2019, 215, 18. [Google Scholar] [CrossRef]
- Ohnishi, I.; Tomeoka, K. Dark inclusions in the Mokoia CV3 chondrite: Evidence for aqueous alteration and subsequent thermal and shock metamorphism. Meteorit. Planet. Sci. 2002, 37, 1843–1856. [Google Scholar] [CrossRef]
- Ciesla, F.J.; Lauretta, D.S.; Cohen, B.A.; Hood, L.L. A Nebular Origin for Chondritic Fine-Grained Phyllosilicates. Science 2003, 299, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B. Modeling of Liquid Water on CM Meteorite Parent Bodies and Implications for Amino Acid Racemization. Icarus 2000, 145, 369–381. [Google Scholar] [CrossRef]
- Scott, E.R.D.; Krot, A.N. Chondrites and Their Components. Treatise Geochem. 2003, 1, 711. [Google Scholar]
- Pignatelli, I.; Mugnaioli, E.; Marrocchi, Y. Cronstedtite polytypes in the Paris meteorite. Eur. J. Miner. 2018, 30, 349–354. [Google Scholar] [CrossRef]
- Meunier, A.; Mas, A.; Beaufort, D.; Patrier, P.; Dudoignon, P. Clay Minerals in Basalt-Hawaiite Rocks From Mururoa Atoll (French Polynesia). II. Petrography and Geochemistry. Clays Clay Miner. 2008, 56, 730–750. [Google Scholar] [CrossRef]
- Mas, A.; Meunier, A.; Beaufort, D.; Patrier, P.; Dudoignon, P. Clay Minerals in Basalt-Hawaiite Rocks From Mururoa Atoll (French Polynesia). I. Mineralogy. Clays Clay Miner. 2008, 56, 711–729. [Google Scholar] [CrossRef]
- Turpault, M.; Meunier, A.; Guilhaumou, N.; Touchard, G. Analysis of hot fluid infiltration in fractured granite by fluid inclusion study. Appl. Geochem. 1992, 7, 269–276. [Google Scholar] [CrossRef]
- Meunier, A.; Sardini, P.; Robinet, J.C.; Prêt, D. The petrography of weathering processes: Facts and outlooks. Clay Miner. 2007, 42, 415–435. [Google Scholar] [CrossRef]
- Banfield, J.F.; Barker, W.W. Direct observation of reactant-product interfaces formed in natural weathering of exsolved, defective amphibole to smectite: Evidence for episodic, isovolumetric reactions involving structural inheritance. Geochim. Cosmochim. Acta 1994, 58, 1419–1429. [Google Scholar] [CrossRef]
- Proust, D.; Caillaud, J.; Fontaine, C. Clay Minerals in Early Amphibole Weathering: Tri- to Dioctahedral Sequence as a Function of Crystallization Sites in the Amphibole. Clays Clay Miner. 2006, 54, 351–362. [Google Scholar] [CrossRef]
- Velbel, M.A. Weathering of Hornblende to Ferruginous Products by a Dissolution-Reprecipitation Mechanism: Petrography and Stoichiometry. Clays Clay Miner. 1989, 37, 515–524. [Google Scholar] [CrossRef]
- Eggleton, R.A.; Boland, J.N. Weathering of Enstatite to Talc through a Sequence of Transitional Phases. Clays Clay Miner. 1982, 30, 11–20. [Google Scholar] [CrossRef]
- Velbel, M.A. Dissolution of olivine during natural weathering. Geochim. Cosmochim. Acta 2009, 73, 6098–6113. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Velbel, M.A.; Barker, W.W. Pyroxene weathering to smectite: Conventional and cryo-field emission scanning electron microscopy, Koua Bocca ultramafic complex, Ivory Coast. Clays Clay Miner. 2008, 56, 112–127. [Google Scholar] [CrossRef]
- Drief, A.; Schiffman, P. Very low-temperature alteration of sideromelane in hyaloclastites and hyalotuffs from Kilauea and Mauna Kea volcanoes: Implications for the mechanism of palagonite formation. Clays Clay Miner. 2004, 52, 622–634. [Google Scholar] [CrossRef]
- Goff, F. Vesicle cylinders in vapor-differentiated basalt flows. J. Volcanol. Geotherm. Res. 1996, 71, 167–185. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Gemmi, M.; Dapiaggi, M. Phase transformations and reaction kinetics during the temperature-induced oxidation of natural olivine. Am. Miner. 2003, 88, 1560–1574. [Google Scholar] [CrossRef]
- Caroff, M.; Maury, R.C.; Cotten, J.; Clément, J.-P. Segregation structures in vapor-differentiated basaltic flows. Bull. Volcanol. 2000, 62, 171–187. [Google Scholar] [CrossRef]
- Clément, J.-P.; Caroff, M.; Dudoignon, P.; Launeau, P.; Bohn, M.; Cotten, J.; Blais, S.; Guille, G. A possible link between gabbros bearing High Temperature Iddingsite alteration and huge pegmatoid intrusions: The Society Islands, French Polynesia. Lithos 2007, 96, 524–542. [Google Scholar] [CrossRef]
- Meunier, A. Clays in Sedimentary Environments. In Clays; Springer: Berlin/Heidelberg, Germany, 2005; pp. 295–327. ISBN 978-3-540-27141-3. [Google Scholar]
- Alt, J.C. Very Low-Grade Hydrothermal Metamorphism of Basic Igneous Rocks. In Low-Grade Metamorphism; Blackwell Pub: Vestavia Hills, AL, USA, 1999; pp. 169–201. [Google Scholar]
- Neuhoff, P.S. Porosity evolution and mineral paragenesis during low-grade metamorphism of basaltic lavas at Teigarhorn, eastern Iceland. Am. J. Sci. 1999, 299, 467–501. [Google Scholar] [CrossRef]
- Goltz, A.E.; Krawczynski, M.J.; Gavrilenko, M.; Gorbach, N.V.; Ruprecht, P. Evidence for superhydrous primitive arc magmas from mafic enclaves at Shiveluch volcano, Kamchatka. Contrib. Miner. Pet. 2020, 175, 1–26. [Google Scholar] [CrossRef]
- Grigor’ev, D.P. Ontogeny of Minerals: Israel Program for Scientific Translations; Davey: Jerusalem, Israel, 1965. [Google Scholar]
- Meunier, A.; Petit, S.; Ehlmann, B.L.; Dudoignon, P.; Westall, F.; Mas, A.; El Albani, A.; Ferrage, E. Magmatic precipitation as a possible origin of Noachian clays on Mars. Nat. Geosci. 2012, 5, 739–743. [Google Scholar] [CrossRef]
- Nakazawa, H.; Yamada, H.; Fujita, T. Crystal synthesis of smectite applying very high pressure and temperature. Appl. Clay Sci. 1992, 6, 395–401. [Google Scholar] [CrossRef]
- Decarreau, A.; Petit, S.; Vieillard, P.; Dabert, N. Hydrothermal synthesis of aegirine at 200C. Eur. J. Miner. 2004, 16, 85–90. [Google Scholar] [CrossRef]
- De Leuw, S.; Rubin, A.E.; Wasson, J.T. Carbonates in CM chondrites: Complex formational histories and comparison to carbonates in CI chondrites. Meteorit. Planet. Sci. 2010, 45, 513–530. [Google Scholar] [CrossRef]
- Tomeoka, K.; Buseck, P.R. Indicators of aqueous alteration in CM carbonaceous chondrites: Microtextures of a layered mineral containing Fe, S, O and Ni. Geochim. Cosmochim. Acta 1985, 49, 2149–2163. [Google Scholar] [CrossRef]
- Tomeoka, K.; McSween, H.Y., Jr.; Buseck, P.R. Mineralogical Alteration of CM Carbonaceous Chondrites: A View. In Proceedings of the NIPR Symposium on Antarctic Meteorites; National Institute of Polar Research: Tachikawa, Japan, 1989; Volume 2, pp. 221–234. [Google Scholar]
- Alexander, C.M.O.; Howard, K.T.; Bowden, R.; Fogel, M.L. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 2013, 123, 244–260. [Google Scholar] [CrossRef]
- Young, E.D.; Zhang, K.K.; Schubert, G. Conditions for pore water convection within carbonaceous chondrite parent bodies – implications for planetesimal size and heat production. Earth Planet. Sci. Lett. 2003, 213, 249–259. [Google Scholar] [CrossRef]
- Krot, A.N.; Hutcheon, I.D.; Brearley, A.J.; Pravdivtseva, O.V.; Petaev, M.I.; Hohenberg, C.M. Timescales and Settings for Alteration of Chondritic Meteorites; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2005.
- Bland, P.A.; Alard, O.; Benedix, G.K.; Kearsley, A.T.; Menzies, O.N.; Watt, L.E.; Rogers, N.W. Volatile fractionation in the early solar system and chondrule/matrix complementarity. Proc. Natl. Acad. Sci. USA 2005, 102, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, N.; Kebukawa, Y.; Furukawa, Y.; Kondo, M.; Nakano, H.; Kobayashi, K. Aqueous alteration without initial water: Possibility of organic-induced hydration of anhydrous silicates in meteorite parent bodies. Earth Planets Space 2021, 73, 1–11. [Google Scholar] [CrossRef]
- Frost, C.D.; von Blanckenburg, F.; Schoenberg, R.; Frost, B.R.; Swapp, S.M. Preservation of Fe isotope heterogeneities during diagenesis and metamorphism of banded iron formation. Contrib. Miner. Pet. 2006, 153, 211–235. [Google Scholar] [CrossRef]
- Velbel, M.A.; Tonui, E.K.; Zolensky, M.E. Replacement of olivine by serpentine in the Queen Alexandra Range 93005 carbonaceous chondrite (CM2): Reactant–product compositional relations, and isovolumetric constraints on reaction stoichiometry and elemental mobility during aqueous alteration. Geochim. Cosmochim. Acta 2015, 148, 402–425. [Google Scholar] [CrossRef]
- Tomeoka, K. Metamorphic Processes in New CI Carbonaceous Chondrites from Antarctica: Mineralogy and Petrology. Primit. Sol. Nebul. Orig. Planets 1993, 447–464. [Google Scholar]
- Dungan, M.A. Metastability in Serpentine-Olivine Equilibria. Am. Mineral. 1977, 62, 1018–1029. [Google Scholar]
- Grove, T.L.; Till, C.B.; Lev, E.; Chatterjee, N.; Médard, E. Kinematic variables and water transport control the formation and location of arc volcanoes. Nature 2009, 459, 694–697. [Google Scholar] [CrossRef]
- Evans, B.W. Control of the Products of Serpentinization by the Fe2+Mg-1 Exchange Potential of Olivine and Orthopyroxene. J. Pet. 2008, 49, 1873–1887. [Google Scholar] [CrossRef]
- Evans, B.W.; Kuehner, S.M.; Chopelas, A. Magnetite-free, yellow lizardite serpentinization of olivine websterite, Canyon Mountain complex, N.E. Oregon. Am. Miner. 2009, 94, 1731–1734. [Google Scholar] [CrossRef]
- Beard, J.S.; Frost, B.R.; Fryer, P.; McCaig, A.; Searle, R.; Ildefonse, B.; Zinin, P.; Sharma, S.K. Onset and Progression of Serpentinization and Magnetite Formation in Olivine-rich Troctolite from IODP Hole U1309D. J. Pet. 2009, 50, 387–403. [Google Scholar] [CrossRef]
- Hicks, L.; Bridges, J.; Gurman, S. Ferric saponite and serpentine in the nakhlite martian meteorites. Geochim. Cosmochim. Acta 2014, 136, 194–210. [Google Scholar] [CrossRef]
- Koizumi, M.; Roy, R. Synthetic Montmorillonoids with Variable Exchange Capacity*. Am. Mineral. 1959, 44, 788–805. [Google Scholar]
- Iiyama, J.T.; Roy, R. Controlled Synthesis of Heteropolytypic (Mixed-Layer) Clay Minerals. Clays Clay Miner. (Natl. Conf. Clays Clay Miner. 1961, 10, 4–22. [Google Scholar] [CrossRef]
- Whitney, G. Hydrothermal Reactivity of Saponite. Clays Clay Miner. 1983, 31, 1–8. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Komarneni, S.; Amonette, J.E. Synthesis of Smectite Clay Minerals: A Critical Review. Clays Clay Miner. 1999, 47, 529–554. [Google Scholar] [CrossRef]
- Rubin, A.; Trigo-Rodríguez, J.; Huber, H.; Wasson, J. Progressive alteration of CM carbonaceous chondrites. Geochim. Cosmochim. Acta 2007, 71, 2361–2382. [Google Scholar] [CrossRef]
- Grimm, R.E.; McSween Jr, H.Y. Water and the Thermal Evolution of Carbonaceous Chondrite Parent Bodies. Icarus 1989, 82, 244–280. [Google Scholar] [CrossRef]
- Ciesla, F.; Lauretta, D. Radial migration and dehydration of phyllosilicates in the solar nebula. Earth Planet. Sci. Lett. 2005, 231, 1–8. [Google Scholar] [CrossRef]
- Schneeberger, A.; Mousis, O.; Deleuil, M.; Lunine, J.I. Formation of the Trappist-1 system in a dry protoplanetary disk. Astron. Astrophys. 2024, 682, L10. [Google Scholar] [CrossRef]
- McSween Jr, H.Y. Aqueous Alteration in Carbonaceous Chondrites: Mass Balance Constraints on Matrix Mineralogy. Geochim. Cosmochim. Acta 1987, 51, 2469–2477. [Google Scholar] [CrossRef]
- Nakato, A.; Nakamura, T.; Kitajima, F.; Noguchi, T. Evaluation of dehydration mechanism during heating of hydrous asteroids based on mineralogical and chemical analysis of naturally and experimentally heated CM chondrites. Earth Planets Space 2008, 60, 855–864. [Google Scholar] [CrossRef]
- Endress, M.; Zinner, E.; Bischoff, A. Early aqueous activity on primitive meteorite parent bodies. Nature 1996, 379, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Paoletti, M.J.; Marrocchi, Y.; Avice, G.; Roskosz, M.; Gurenko, A.; Gounelle, M. Oxygen isotope constraints on the alteration temperatures of CM chondrites. Earth Planet. Sci. Lett. 2017, 458, 273–281. [Google Scholar] [CrossRef]
- Zolotov, M.Y.; Mironenko, M.V.; Shock, E.L. Thermodynamic constraints on fayalite formation on parent bodies of chondrites. Meteorit. Planet. Sci. 2006, 41, 1775–1796. [Google Scholar] [CrossRef]
- Zheng, Y.-F. “Calculation of Oxygen Isotope Fractionation in Anhydrous Silicate Minerals.” Geochimica et Cosmochimica Acta. Geochim. Cosmochim. Acta 1993, 57, 3199. [Google Scholar] [CrossRef]
- Doyle, P.M.; Jogo, K.; Nagashima, K.; Krot, A.N.; Wakita, S.; Ciesla, F.J.; Hutcheon, I.D. Early aqueous activity on the ordinary and carbonaceous chondrite parent bodies recorded by fayalite. Nat. Commun. 2015, 6, 7444. [Google Scholar] [CrossRef] [PubMed]
- Krot, A.N.; Brearley, A.J.; Petaev, M.I.; Kallemeyn, G.W.; Sears, D.W.; Benoit, P.H.; Hutcheon, I.D.; Zolensky, M.E.; Keil, K. Evidence for Low-Temperature Growth of Fayalite and Hedenbergite in MacAlpine Hills 88107, an Ungrouped Carbonaceous Chondrite Related to the CM-CO Clan. Meteorit. Planet. Sci. 2000, 35, 1365–1386. [Google Scholar] [CrossRef]
- Jogo, K.; Nakamura, T.; Noguchi, T.; Zolotov, M.Y. Fayalite in the Vigarano CV3 carbonaceous chondrite: Occurrences, formation age and conditions. Earth Planet. Sci. Lett. 2009, 287, 320–328. [Google Scholar] [CrossRef]
- Rasmussen, M.G.; Evans, B.W.; Kuehner, S.M. Low-Temperature Fayalite, Greenalite, and Minnesotaite from the Overlook Gold Deposit, Washington; Phase Relations in the System FeO-SiO2-H2O. Can. Mineral. 1998, 36, 147–162. [Google Scholar]
- Goff, B.H.; Weinberg, R.; Groves, D.I.; Vielreicher, N.M.; Fourie, P.J. The Giant Vergenoeg Fluorite Deposit in a Magnetite-Fluorite-Fayalite REE Pipe: A Hydrothermally-Altered Carbonatite-Related Pegmatoid? Mineral. Petrol. 2004, 80, 173. [Google Scholar] [CrossRef]
- Maeda, M.; Tomeoka, K.; Seto, Y. Early aqueous alteration process in the QUE97990 and Y791198 CM carbonaceous chondrites. J. Miner. Pet. Sci. 2009, 104, 92–96. [Google Scholar] [CrossRef]
- Zhou, W.; Peacor, D.R.; Alt, J.C.; Van der Voo, R.; Kao, L.-S. TEM study of the alteration of interstitial glass in MORB by inorganic processes. Chem. Geol. 2001, 174, 365–376. [Google Scholar] [CrossRef]
- Bouquain, S.; Arndt, N.T.; Hellebrand, E.; Faure, F. Crystallochemistry and origin of pyroxenes in komatiites. Contrib. Miner. Pet. 2009, 158, 599–617. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. The origin of spinifex texture in komatiites. Nature 1999, 397, 691–694. [Google Scholar] [CrossRef]
- Rietmeijer, F.J.; Nuth, J.A.; Rochette, P.; Marfaing, J.; Pun, A.; Karner, J.M. Deep metastable eutectic condensation in Al-Fe-SiO-H2-O2 vapors: Implications for natural Fe-aluminosilicates. Am. Miner. 2006, 91, 1688–1698. [Google Scholar] [CrossRef]
- Chizmadia, L.J.; Brearley, A.J. Mineralogy, aqueous alteration, and primitive textural characteristics of fine-grained rims in the Y-791198 CM2 carbonaceous chondrite: TEM observations and comparison to ALHA81002. Geochim. Cosmochim. Acta 2008, 72, 602–625. [Google Scholar] [CrossRef]
- Zanetta, P.-M.; Leroux, H.; Le Guillou, C.; Zanda, B.; Hewins, R. Nebular thermal processing of accretionary fine-grained rims in the Paris CM chondrite. Geochim. Cosmochim. Acta 2020, 295, 135–154. [Google Scholar] [CrossRef]
- Haenecour, P.; Floss, C.; Zega, T.J.; Croat, T.K.; Wang, A.; Jolliff, B.L.; Carpenter, P. Presolar silicates in the matrix and fine-grained rims around chondrules in primitive CO3.0 chondrites: Evidence for pre-accretionary aqueous alteration of the rims in the solar nebula. Geochim. Cosmochim. Acta 2018, 221, 379–405. [Google Scholar] [CrossRef]
- Leitner, J.; Vollmer, C.; Floss, C.; Zipfel, J.; Hoppe, P. Ancient stardust in fine-grained chondrule dust rims from carbonaceous chondrites. Earth Planet. Sci. Lett. 2016, 434, 117–128. [Google Scholar] [CrossRef]
- Sears, D.W.G.; Benoit, P.H.; Jie, L. Two chondrule groups each with distinctive rims in Murchison recognized by cathodoluminescence. Meteoritics 1993, 28, 669–675. [Google Scholar] [CrossRef]
- Takayama, A.; Tomeoka, K. Fine-grained rims surrounding chondrules in the Tagish Lake carbonaceous chondrite: Verification of their formation through parent-body processes. Geochim. Cosmochim. Acta 2012, 98, 1–18. [Google Scholar] [CrossRef]
- Tomeoka, K.; Ohnishi, I. Olivine-rich rims surrounding chondrules in the Mokoia CV3 carbonaceous chondrite: Further evidence for parent-body processes. Geochim. Cosmochim. Acta 2014, 137, 18–34. [Google Scholar] [CrossRef]
- Metzler, K.; Bischoff, A.; Stöffler, D. Accretionary dust mantles in CM chondrites: Evidence for solar nebula processes. Geochim. Cosmochim. Acta 1992, 56, 2873–2897. [Google Scholar] [CrossRef]
- Lauretta, D.S.; Hua, X.; Buseck, P.R. Mineralogy of fine-grained rims in the alh 81002 cm chondrite. Geochim. Cosmochim. Acta 2000, 64, 3263–3273. [Google Scholar] [CrossRef]
- Zolensky, M.; Barrett, R.; Browning, L. Mineralogy and composition of matrix and chondrule rims in carbonaceous chondrites. Geochim. Cosmochim. Acta 1993, 57, 3123–3148. [Google Scholar] [CrossRef]
- Tomeoka, K.; Tanimura, I. Phyllosilicate-rich chondrule rims in the vigarano cv3 chondrite: Evidence for parent-body processes. Geochim. Cosmochim. Acta 2000, 64, 1971–1988. [Google Scholar] [CrossRef]
- Hanowski, N.P.; Brearley, A.J. Aqueous alteration of chondrules in the CM carbonaceous chondrite, Allan Hills 81002: Implications for parent body alteration. Geochim. Cosmochim. Acta 2001, 65, 495–518. [Google Scholar] [CrossRef]
- Lauretta, D.S.; Buseck, P.R. Opaque Minerals in Chondrules and Fine-Grained Chondrule Rims in the Bishunpur (LL3. 1) Chondrite. Meteorit. Planet. Sci. 2003, 38, 59–79. [Google Scholar] [CrossRef]
- Alexander, C. Trace element contents of chondrule rims and interchondrule matrix in ordinary chondrites. Geochim. Cosmochim. Acta 1995, 59, 3247–3266. [Google Scholar] [CrossRef]
- Noguchi, T.; Nakamura, T.; Nozaki, W. Mineralogy of phyllosilicate-rich micrometeorites and comparison with Tagish Lake and Sayama meteorites. Earth Planet. Sci. Lett. 2002, 202, 229–246. [Google Scholar] [CrossRef]
- Graham, G.A.; Bradley, J.P.; Bernas, M.; Stroud, R.M.; Dai, Z.R.; Floss, C.; Stadermann, F.J.; Snead, C.J.; Westphal, A.J. Focused Ion Beam Recovery and Analysis of Interplanetary Dust Particles (IDPs) and Stardust Analogues. Lunar and Planetary Science XXXV: Interplanetary Dust and Aerogel; NASA: Washington, DC, USA, 2004.
- Morris, M.A.; Desch, S.J. Phyllosilicate Emission from Protoplanetary Disks: Is the Indirect Detection of Extrasolar Water Possible? Astrobiology 2009, 9, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Deloule, E.; Robert, F. Interstellar Water in Meteorites? Geochim. Cosmochim. Acta 1995, 59, 4695–4706. [Google Scholar] [CrossRef] [PubMed]
- Richard A áCatlow, C.; Helen, E. Where on Earth Has Our Water Come From? Chem. Commun. 2010, 46, 8923–8925. [Google Scholar]
- Wasson, J.T.; Rubin, A.E. Composition of matrix in the CR chondrite LAP 02342. Geochim. Cosmochim. Acta 2009, 73, 1436–1460. [Google Scholar] [CrossRef]
- Cohen, B.A.; Hewins, R.H.; Alexander, C.M. The formation of chondrules by open-system melting of nebular condensates 1 1Associate editor: C. Koeberl. Geochim. Cosmochim. Acta 2004, 68, 1661–1675. [Google Scholar] [CrossRef]
- Rubin, A.E.; Wasson, J.T. Non-Spherical Lobate Chondrules in CO3. 0 Y-81020: General Implications for the Formation of Low-FeO Porphyritic Chondrules in CO Chondrites. Geochim. Cosmochim. Acta 2005, 69, 211–220. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Zhigilei, L.V. Effect of Pressure Relaxation on the Mechanisms of Short-Pulse Laser Melting. Phys. Rev. Lett. 2003, 91, 105701. [Google Scholar] [CrossRef]
- Ivanov, D.S.; Zhigilei, L.V. Kinetic Limit of Heterogeneous Melting in Metals. Phys. Rev. Lett. 2007, 98, 195701. [Google Scholar] [CrossRef]
- Auxerre, M.; Faure, F.; Lequin, D. The effects of superheating and cooling rate on olivine growth in chondritic liquid. Meteorit. Planet. Sci. 2022, 57, 1474–1495. [Google Scholar] [CrossRef]
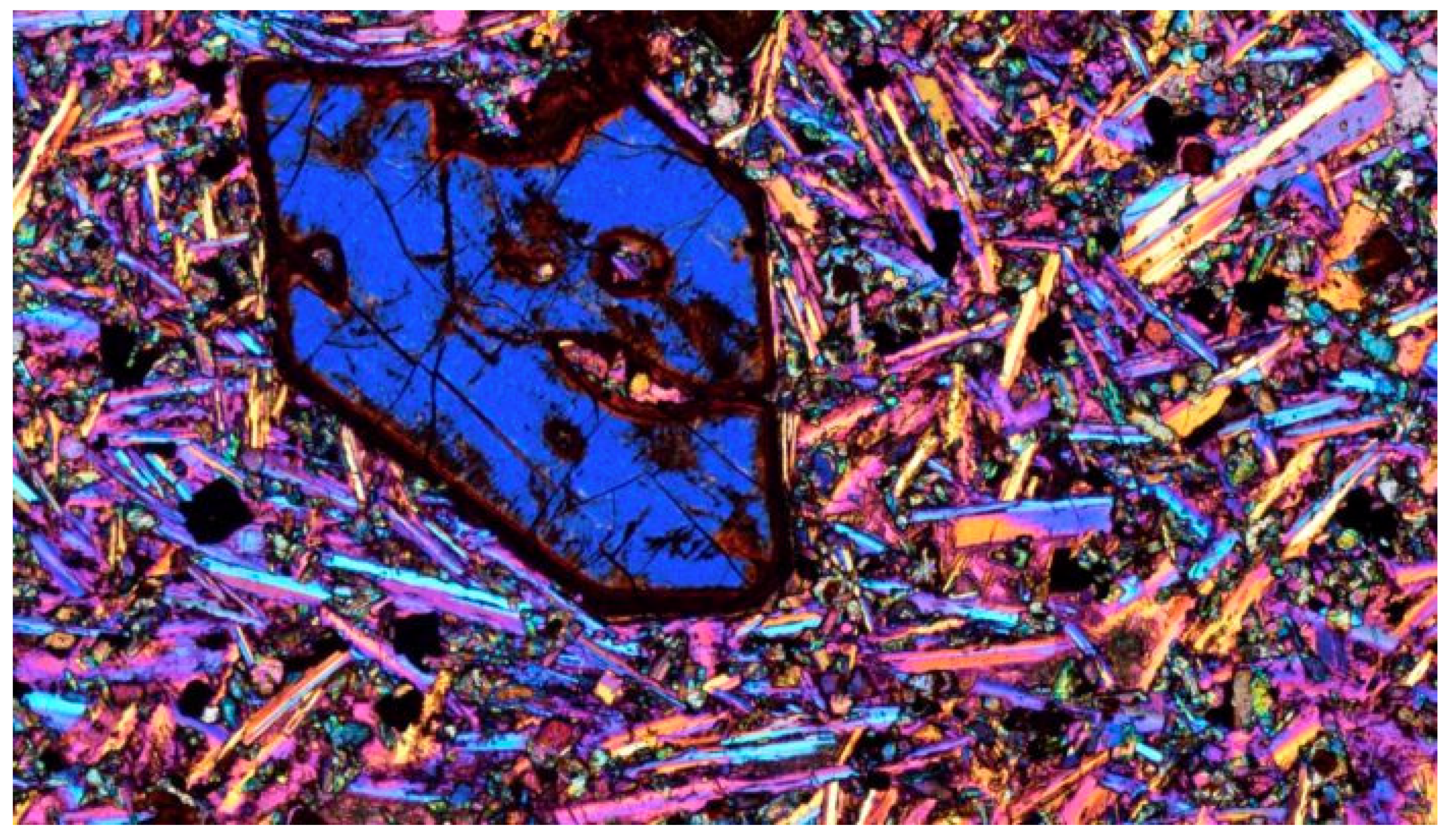
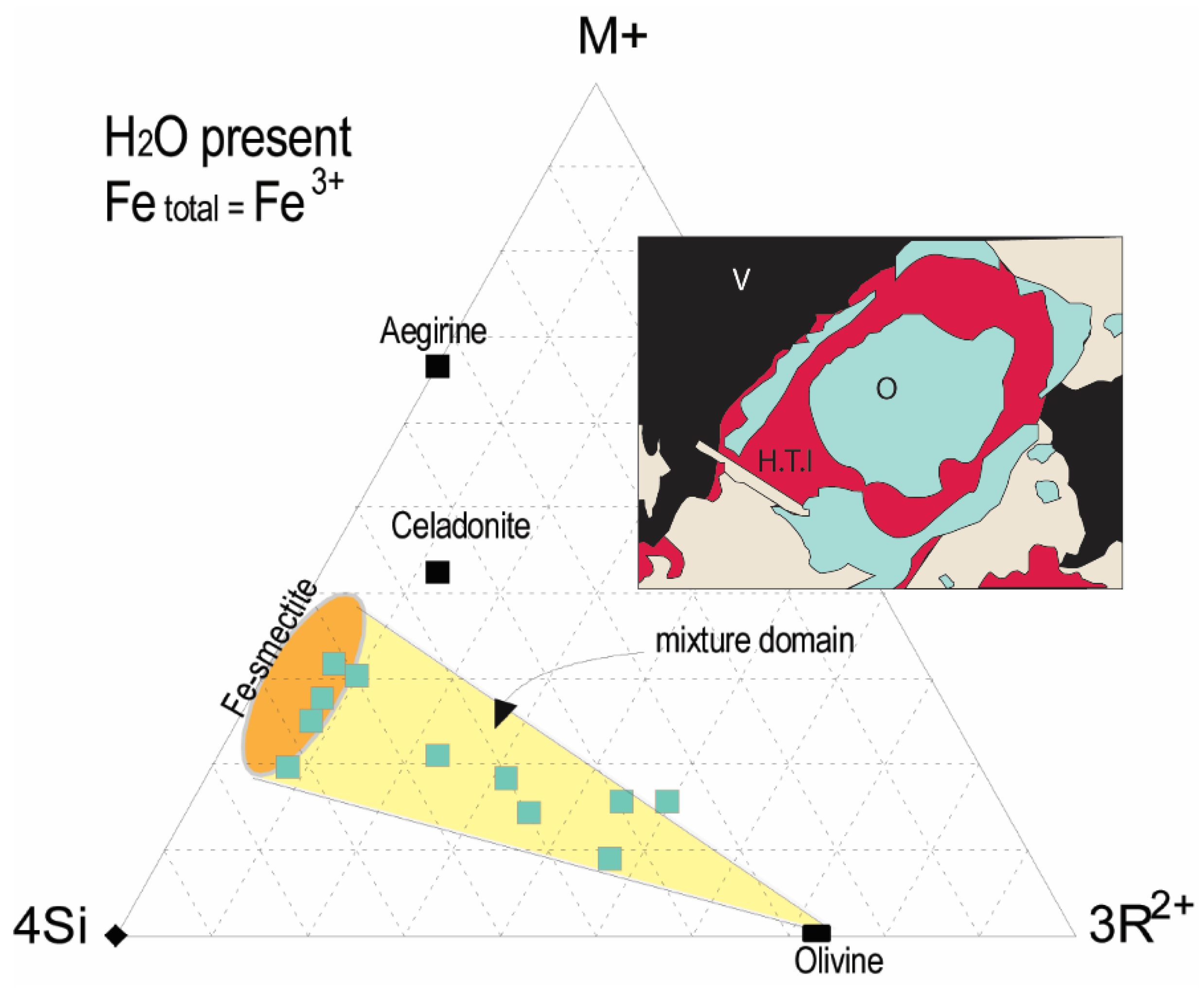





| Embayments | Rims | ||
|---|---|---|---|
| Tochilinite-Rich | Cronstedtite-Rich | Mg-Fe Serpentine-Rich | |
| Number of Analysis | 20 | 21 | 35 |
| SiO2 | 3.47 (±0.99) | 20.3 (±3.3) | 24.3 (±1.9) |
| TiO2 | 0.14 (±0.05) | 0.11 (±0.09) | 0.11 (±0.09) |
| Al2O3 | 0.55 (±0.14) | 3.98 (±0.87) | 2.87 (±0.48) |
| Cr2O3 | 1.78 (±1.09) | 0.61 (±0.92) | 0.44 (±0.17) |
| FeO | 46.1 (±2.9) | 40.6 (±11.4) | 25.2 (±1.7) |
| NiO | 11.1 (±1.7) | 2.09 (±1.62) | 2.59 (±0.54) |
| MnO | 1.15 (±0.33) | 1.00 (±0.39) | 0.18 (±0.21) |
| MgO | 3.83 (±0.47) | 8.91 (±2.93) | 14.4 (±1.8) |
| CaO | 0.73 (±0.17) | 0.50 (±0.50) | 1.03 (±0.28) |
| Na2O | 0.13 (±0.07) | 0.36 (±0.23) | 0.16 (±0.12) |
| S | 15.6 (±1.1) | 2.92 (±1.48) | 4.08 (±0.70) |
| Total | 84.6 | 84.4 | 75.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Albani, A.; Chraiki, I.; Aoudjehane, H.C.; Ghnahalla, M.; Abdelfadel, F.; Elmola, A.A.; Bankole, O.; Ikouanga, J.N.; El Khoury, A.; Fontaine, C.; et al. Are Clay Minerals Systematically the Products of Aqueous Alteration in Cosmic Bodies? Minerals 2024, 14, 486. https://doi.org/10.3390/min14050486
El Albani A, Chraiki I, Aoudjehane HC, Ghnahalla M, Abdelfadel F, Elmola AA, Bankole O, Ikouanga JN, El Khoury A, Fontaine C, et al. Are Clay Minerals Systematically the Products of Aqueous Alteration in Cosmic Bodies? Minerals. 2024; 14(5):486. https://doi.org/10.3390/min14050486
Chicago/Turabian StyleEl Albani, Abderrazak, Ibtissam Chraiki, Hasnaa Chennaoui Aoudjehane, Mohamed Ghnahalla, Fatima Abdelfadel, Ahmed Abd Elmola, Olabode Bankole, Julie Ngwal’ghoubou Ikouanga, Anna El Khoury, Claude Fontaine, and et al. 2024. "Are Clay Minerals Systematically the Products of Aqueous Alteration in Cosmic Bodies?" Minerals 14, no. 5: 486. https://doi.org/10.3390/min14050486





