Potential Utilization of Loess in Grouting Materials: Effects of Grinding Time and Calcination Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Grinding and Calcination of Loess
2.3. Sample Preparation
2.4. Methods
3. Results and Discussion
3.1. Grinding Modification
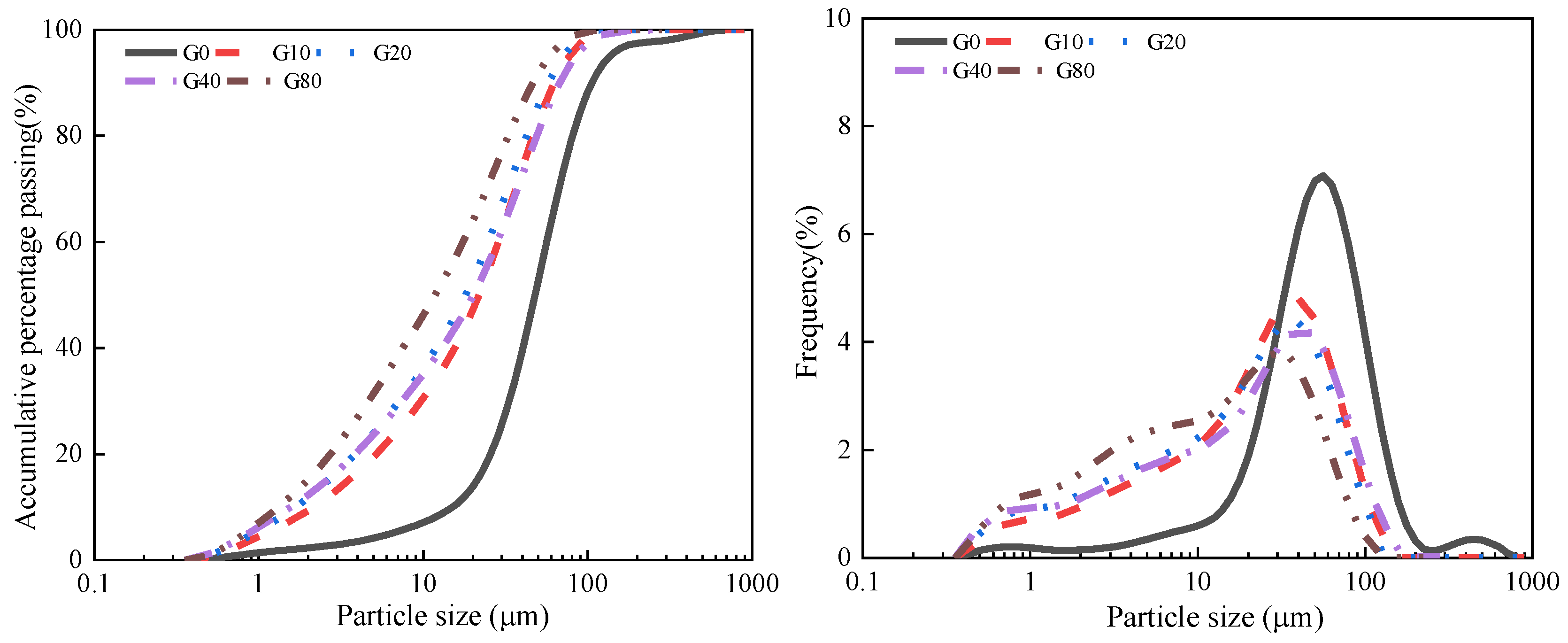
3.1.1. Particle Size Analysis
3.1.2. Pozzolanic Activity Analysis
3.2. Calcination Modification
3.2.1. Mineral Phase Analysis
3.2.2. Pozzolanic Activity Analysis
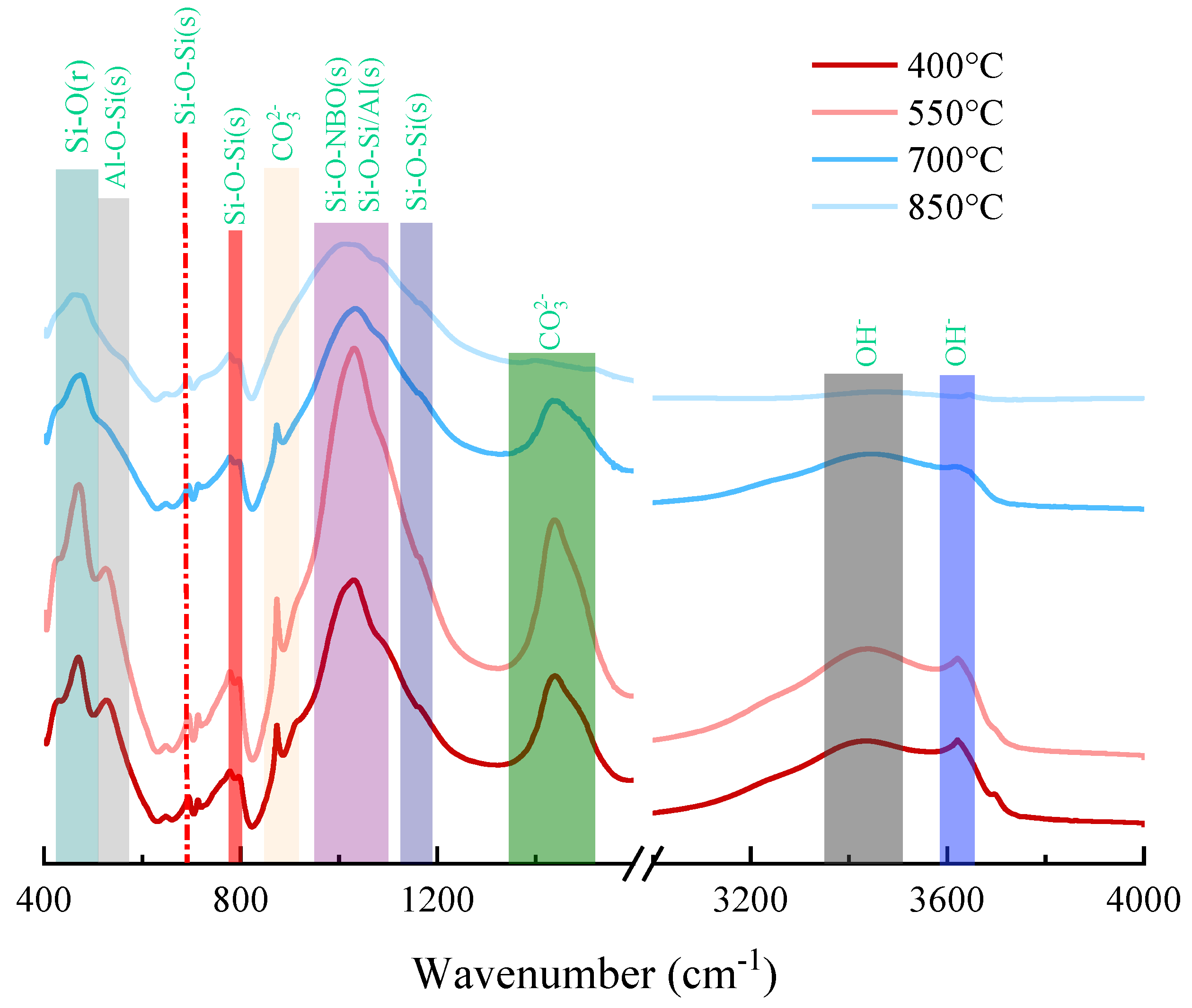
3.2.3. FTIR Analysis
3.3. Workability of Novel Grouting Materials
3.3.1. Setting Time
3.3.2. Fluidity Behavior
3.3.3. UCS and TG Analyses
4. Conclusions
- (1)
- Loess decreases in particle size and increases in ultrafine particles with increasing grinding time, which destroys the internal structure of the highly crystalline loess and increases the contact points of the alkaline reaction and the degree of disorder in Si and Al, thereby improving the pozzolanic activity. The increase in ultrafine particles after further grinding leads to agglomeration, forming porous spherical aggregates.
- (2)
- The pozzolanic activity of modified loess increases with the increase in calcination temperature. At 550 °C, the free and bound water of loess was lost. At 850 °C, anorthite and muscovite decomposed and their peak intensities decreased. The vibrations in the 400–500 cm−1 spectral band indicated that the destruction of the Si–O covalent bond at high temperatures produced Si-phases. The spectral bands of ~900 cm−1 and ~1400 cm−1 corresponded to the vibrations of the C–O bond in CO32−, indicating that carbonate partially decomposed at 850 °C.
- (3)
- Increasing the grinding time reduces the fluidity and increases the setting time of loess. As an auxiliary cementitious material, this can improve the uniaxial compressive strength. As the calcination temperature increases, the fluidity and setting time decrease. Simultaneously, the pozzolanic activity is improved, which promotes the formation of C–S–H gel and ettringite and increases the compressive strength.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korkmaz, A.V. Mechanical activation of diabase and its effect on the properties and microstructure of Portland cement. Case Stud. Constr. Mater. 2022, 16, e00868. [Google Scholar] [CrossRef]
- Baki, V.A.; Ke, X.; Heath, A.; Calabria-Holley, J.; Terzi, C.; Sirin, M. The impact of mechanochemical activation on the physicochemical properties and pozzolanic reactivity of kaolinite, muscovite and montmorillonite. Cem. Concr. Res. 2022, 162, 106962. [Google Scholar] [CrossRef]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C.R. Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cem. Concr. Compos. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Tole, I.; Delogu, F.; Qoku, E.; Habermehl-Cwirzen, K.; Cwirzen, A. Enhancement of the pozzolanic activity of natural clays by mechanochemical activation. Constr. Build. Mater. 2022, 352, 128739. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Q.; Wang, J.; Wu, P.; Lyu, X. Effect of mechanical grinding on pozzolanic activity and hydration properties of siliceous gold ore tailings. J. Clean. Prod. 2019, 217, 12–21. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Z.; Yao, J.; Cong, X.; Anning, C.; Lyu, X. Pozzolanic activity and hydration properties of feldspar after mechanical activation. Powder Technol. 2021, 383, 167–174. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Zhang, J.; Wang, J.; Lyu, X. Effects of mechanical grinding on pozzolanic activity and hydration properties of quartz. Adv. Powder Technol. 2020, 31, 4500–4509. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Tian, Y.; Wu, P.; Guo, Z.; Qiu, J.; Xing, J.; Xiaowei, G. Modification of high-volume fly ash cement with metakaolin for its utilization in cemented paste backfill: The effects of metakaolin content and particle size. Powder Technol. 2021, 393, 539–549. [Google Scholar] [CrossRef]
- Andrić, L.; Terzić, A.; Aćimović-Pavlović, Z.; Pavlović, L.; Petrov, M. Comparative kinetic study of mechanical activation process of mica and talc for industrial application. Compos. Part B Eng. 2014, 59, 181–190. [Google Scholar] [CrossRef]
- Yao, G.; Zang, H.; Wang, J.; Wu, P.; Qiu, J.; Lyu, X. Effect of Mechanical Activation on the Pozzolanic Activity of Muscovite. Clays Clay Miner. 2019, 67, 209–216. [Google Scholar] [CrossRef]
- Mitrović, A.; Zdujić, M. Preparation of pozzolanic addition by mechanical treatment of kaolin clay. Int. J. Miner. Process. 2014, 132, 59–66. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Increasing the kaolinite content of raw clays using particle classification techniques for use as supplementary cementitious materials. Constr. Build. Mater. 2020, 244, 118335. [Google Scholar] [CrossRef]
- Tole, I.; Habermehl-Cwirzen, K.; Cwirzen, A. Mechanochemical activation of natural clay minerals: An alternative to produce sustainable cementitious binders—Review. Mineral. Petrol. 2019, 113, 449–462. [Google Scholar] [CrossRef]
- Neißer-Deiters, A.; Scherb, S.; Beuntner, N.; Thienel, K.-C. Influence of the calcination temperature on the properties of a mica mineral as a suitability study for the use as SCM. Appl. Clay Sci. 2019, 179, 105168. [Google Scholar] [CrossRef]
- Irassar, E.F.; Bonavetti, V.L.; Castellano, C.C.; Trezza, M.A.; Rahhal, V.F.; Cordoba, G.; Lemma, R. Calcined illite-chlorite shale as supplementary cementing material: Thermal treatment, grinding, color and pozzolanic activity. Appl. Clay Sci. 2019, 179, 105143. [Google Scholar] [CrossRef]
- Hao, R.; Li, X.; Xu, P.; Liu, Q. Thermal activation and structural transformation mechanism of kaolinitic coal gangue from Jungar coalfield, Inner Mongolia, China. Appl. Clay Sci. 2022, 223, 106508. [Google Scholar] [CrossRef]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Rakhimov, R.Z.; Rakhimova, N.R.; Gaifullin, A.R.; Morozov, V.P. Properties of Portland cement pastes enriched with addition of calcined marl. J. Build. Eng. 2017, 11, 30–36. [Google Scholar] [CrossRef]
- Sun, T.; Ge, K.; Wang, G.; Geng, H.; Shui, Z.; Cheng, S.; Chen, M. Comparing pozzolanic activity from thermal-activated water-washed and coal-series kaolin in Portland cement mortar. Constr. Build. Mater. 2019, 227, 117092. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Lin, M.; Xia, Z.; Pei, Z.; Li, B. Influence of calcined coal-series kaolin fineness on properties of cement paste and mortar. Constr. Build. Mater. 2018, 171, 558–565. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S. Reexamining calcination of kaolinite for the synthesis of metakaolin geopolymers—Roles of dehydroxylation and recrystallization. J. Non-Cryst. Solids 2017, 460, 74–80. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays: Factors affecting its performance as pozzolans. Constr. Build. Mater. 2012, 28, 276–281. [Google Scholar] [CrossRef]
- Claverie, M.; Martin, F.; Tardy, J.P.; Cyr, M.; De Parseval, P.; Grauby, O.; Le Roux, C. Structural and chemical changes in kaolinite caused by flash calcination: Formation of spherical particles. Appl. Clay Sci. 2015, 114, 247–255. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Xu, X.; Lao, X.; Wu, J.; Zhang, Y.; Xu, X.; Li, K. Microstructural evolution, phase transformation, and variations in physical properties of coal series kaolin powder compact during firing. Appl. Clay Sci. 2015, 115, 76–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Seetharaman, S.; Liu, L.; Wang, X.; Zhang, Z. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2014, 48, 2779–2793. [Google Scholar] [CrossRef]
- Lee, V.-G.; Yeh, T.-H. Sintering effects on the development of mechanical properties of fired clay ceramics. Mater. Sci. Eng. A 2008, 485, 5–13. [Google Scholar] [CrossRef]
- Cheng, S.; Ge, K.; Sun, T.; Shui, Z.; Chen, X.; Lu, J.-X. Pozzolanic activity of mechanochemically and thermally activated coal-series kaolin in cement-based materials. Constr. Build. Mater. 2021, 299, 123972. [Google Scholar] [CrossRef]
- Vizcayno, C.; de Gutiérrez, R.M.; Castello, R.; Rodriguez, E.; Guerrero, C.E. Pozzolan obtained by mechanochemical and thermal treatments of kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- Guo, W.; Li, D.; Chen, J.; Yang, N. Structure and pozzolanic activity of calcined coal gangue during the process of mechanical activation. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 326–329. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Souri, A.; Kazemi-Kamyab, H.; Snellings, R.; Naghizadeh, R.; Golestani-Fard, F.; Scrivener, K. Pozzolanic activity of mechanochemically and thermally activated kaolins in cement. Cem. Concr. Res. 2015, 77, 47–59. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Zhao, Y.; Qiu, J.; Guo, Z. The effect of stone waste on the properties of cemented paste backfill using alkali-activated slag as binder. Constr. Build. Mater. 2021, 283, 122686. [Google Scholar] [CrossRef]
- Sonebi, M.; Lachemi, M.; Hossain, K.M.A. Optimisation of rheological parameters and mechanical properties of superplasticised cement grouts containing metakaolin and viscosity modifying admixture. Constr. Build. Mater. 2013, 38, 126–138. [Google Scholar] [CrossRef]
- Singh, M.; Garg, M. Reactive pozzolana from Indian clays—Their use in cement mortars. Cem. Concr. Res. 2006, 36, 1903–1907. [Google Scholar] [CrossRef]
- Paiva, H.; Velosa, A.; Cachim, P.; Ferreira, V.M. Effect of metakaolin dispersion on the fresh and hardened state properties of concrete. Cem. Concr. Res. 2012, 42, 607–612. [Google Scholar] [CrossRef]
- Cassagnabère, F.; Diederich, P.; Mouret, M.; Escadeillas, G.; Lachemi, M. Impact of metakaolin characteristics on the rheological properties of mortar in the fresh state. Cem. Concr. Compos. 2013, 37, 95–107. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Li, Y.; Li, Z.; Zhao, Y. Mechanical, mineralogical, and microstructural characterization of collapsible loess cured by NaOH solution. Constr. Build. Mater. 2024, 421, 135678. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, C.; Wen, J.; Guo, H.; Fan, H. Mechanical characterization and water stability of loess improved by bio-based materials: An eco-friendly approach. Sci. Total Environ. 2024, 921, 171111. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Gu, X.; Nehdi, M.L.; Zhang, L.V. Mechanochemical activation of iron ore tailing-based ternary supplementary cementitious materials. Constr. Build. Mater. 2022, 346, 128420. [Google Scholar] [CrossRef]
- Chen, B.; Pang, L.; Zhou, Z.; Chang, Q.; Fu, P. Study on the activation mechanism and hydration properties of gold tailings activated by mechanical-chemical-thermal coupling. J. Build. Eng. 2022, 48, 104014. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Zhang, Z. Pozzolanic activity evaluation methods of solid waste: A review. J. Clean. Prod. 2023, 402, 136783. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Guo, Z.; Zhang, S.; Wu, P.; Sun, X. Activation the hydration properties of illite-containing tailings to prepare a binder for cemented paste backfill. Constr. Build. Mater. 2022, 318, 125989. [Google Scholar] [CrossRef]
- Qiu, Z.; Bao, S.; Zhang, Y.; Huang, M.; Lin, C.; Huang, X.; Chen, Y.; Ping, Y. Effect of Portland cement on the properties of geopolymers prepared from granite powder and fly ash by alkali-thermal activation. J. Build. Eng. 2023, 76, 107363. [Google Scholar] [CrossRef]
- Yanguatin, H.; Ramírez, J.H.; Tironi, A.; Tobón, J.I. Effect of thermal treatment on pozzolanic activity of excavated waste clays. Constr. Build. Mater. 2019, 211, 814–823. [Google Scholar] [CrossRef]
- Derouiche, R.; Baklouti, S. Phosphoric acid based geopolymerization: Effect of the mechanochemical and the thermal activation of the kaolin. Ceram. Int. 2021, 47, 13446–13456. [Google Scholar] [CrossRef]
- d’Azevedo, C.A.; de Assis, T.C.; Silva, F.A.N.G.; Siqueira, J.M.; Garrido, F.M.S.; Medeiros, M.E. Preparation of α-cordierite through mechanochemical activation of MgO–Al2O3–SiO2 ternary system. Ceram. Int. 2022, 48, 18658–18666. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Su, Y.; He, X.; Ma, B.; Wang, Y.; Li, Y.; Qi, H.; Wang, B. Effect of different retarders on setting time and mechanical properties of hemihydrate phosphogypsum-calcium sulfoaluminate cement composite binder. Constr. Build. Mater. 2024, 411, 134339. [Google Scholar] [CrossRef]
- Ng, Y.L.; Aldahdooh, M.A.A.; Alazaiza, M.Y.D.; Bashir, M.J.K.; Chok, V.S.; Ng, C.A. Influence of alum sludge ash and ground granulated blast furnace slag on properties of cement mortar. Clean. Eng. Technol. 2022, 6, 100376. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J. Clean. Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- Ouyang, S.; Huang, Y.; Wu, L.; Yin, W.; Yang, X.; Wang, J.; Wang, G.; Li, J.; Lei, Y. Effects of chlorides on setting time, hydration heat and hydration products of fresh slurry of cemented paste backfill. Case Stud. Constr. Mater. 2022, 17, e01462. [Google Scholar] [CrossRef]
- Guo, Z.; Qiu, J.; Jiang, H.; Zhang, S.; Ding, H. Improving the performance of superfine-tailings cemented paste backfill with a new blended binder. Powder Technol. 2021, 394, 149–160. [Google Scholar] [CrossRef]
- Hu, Y.; Li, K.; Zhang, B.; Han, B. Effect of nano-SiO2 on mechanical properties, fluidity, and microstructure of superfine tailings cemented paste backfill. Mater. Today Sustain. 2023, 24, 100490. [Google Scholar] [CrossRef]
- Dong, D.; Huang, Y.; Pei, Y.; Zhang, X.; Cui, N.; Zhao, P.; Hou, P.; Lu, L. Effect of spherical silica fume and fly ash on the rheological property, fluidity, setting time, compressive strength, water resistance and drying shrinkage of magnesium ammonium phosphate cement. J. Build. Eng. 2023, 63, 105484. [Google Scholar] [CrossRef]
- Shao, C.; Liu, L.; Zhang, X.; Xie, L.; Ruan, S.; Zhu, M.; Yang, P.; Liu, D. Influence of internal and external factors on the fluidity of modified magnesium slag-based backfill materials. J. Environ. Chem. Eng. 2024, 12, 111867. [Google Scholar] [CrossRef]
- Kaze, C.R.; Adesina, A.; Lecomte-Nana, G.L.; Alomayri, T.; Kamseu, E.; Melo, U.C. Alkali-activated laterite binders: Influence of silica modulus on setting time, Rheological behaviour and strength development. Clean. Eng. Technol. 2021, 4, 100175. [Google Scholar] [CrossRef]
- Kaze, C.R.; Lecomte-Nana, G.L.; Adesina, A.; Nemaleu, J.G.D.; Kamseu, E.; Chinje Melo, U. Influence of mineralogy and activator type on the rheology behaviour and setting time of laterite based geopolymer paste. Cem. Concr. Compos. 2022, 126, 104345. [Google Scholar] [CrossRef]
- Hernández-Ramos, S.M.; Trejo-Arroyo, D.L.; Cholico-González, D.F.; Rodríguez-Torres, G.M.; Zárate-Medina, J.; Vega-Azamar, R.E.; León-Patiño, C.A.; Ortíz-Lara, N. Characterization and effect of mechanical and thermal activation in mining tailings for use as supplementary cementitious material. Case Stud. Constr. Mater. 2024, 20, e02770. [Google Scholar] [CrossRef]
- Makó, É.; Őze, C. The effects of silica fume and diatomaceous earth on the mechanochemical activation and pozzolanic activity of kaolin. Appl. Clay Sci. 2022, 228, 106636. [Google Scholar] [CrossRef]
- Hosseini, S.; Brake, N.A.; Nikookar, M.; Günaydın-Şen, Ö.; Snyder, H.A. Mechanochemically activated bottom ash-fly ash geopolymer. Cem. Concr. Compos. 2021, 118, 103976. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Pekarkova, J.; Nied, D. Novel SCM produced by the co-calcination of aluminosilicates with dolomite. Cem. Concr. Res. 2020, 134, 106083. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Jia, Y.; She, W.; Liu, G.; Yang, Y.; Rong, Z.; Sun, W. The influence of chemical admixtures on the strength and hydration behavior of lime-based composite cementitious materials. Cem. Concr. Compos. 2019, 103, 353–364. [Google Scholar] [CrossRef]
- Wang, H.; Hou, P.; Li, Q.; Adu-Amankwah, S.; Chen, H.; Xie, N.; Zhao, P.; Huang, Y.; Wang, S.; Cheng, X. Synergistic effects of supplementary cementitious materials in limestone and calcined clay-replaced slag cement. Constr. Build. Mater. 2021, 282, 122648. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Huang, B.; Yang, Q.; Li, J. Comparison of mechanical, chemical, and thermal activation methods on the utilisation of recycled concrete powder from construction and demolition waste. J. Build. Eng. 2022, 61, 105295. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, F.; Ding, H.; Qiu, J.; Tian, Y.; Liu, N. Recycling aluminum dross as a mineral admixture in CaO-activated superfine slag. Constr. Build. Mater. 2021, 279, 122434. [Google Scholar] [CrossRef]
- Kaze, C.R.; Beleuk à Moungam, L.M.; Sontia Metekong, J.V.; Alomayri, T.S.; Naghizadeh, A.; Tchadjie, L. Thermal behaviour, microstructural changes and mechanical properties of alkali-activated volcanic scoria-fired waste clay brick blends. Dev. Built Environ. 2023, 14, 100153. [Google Scholar] [CrossRef]





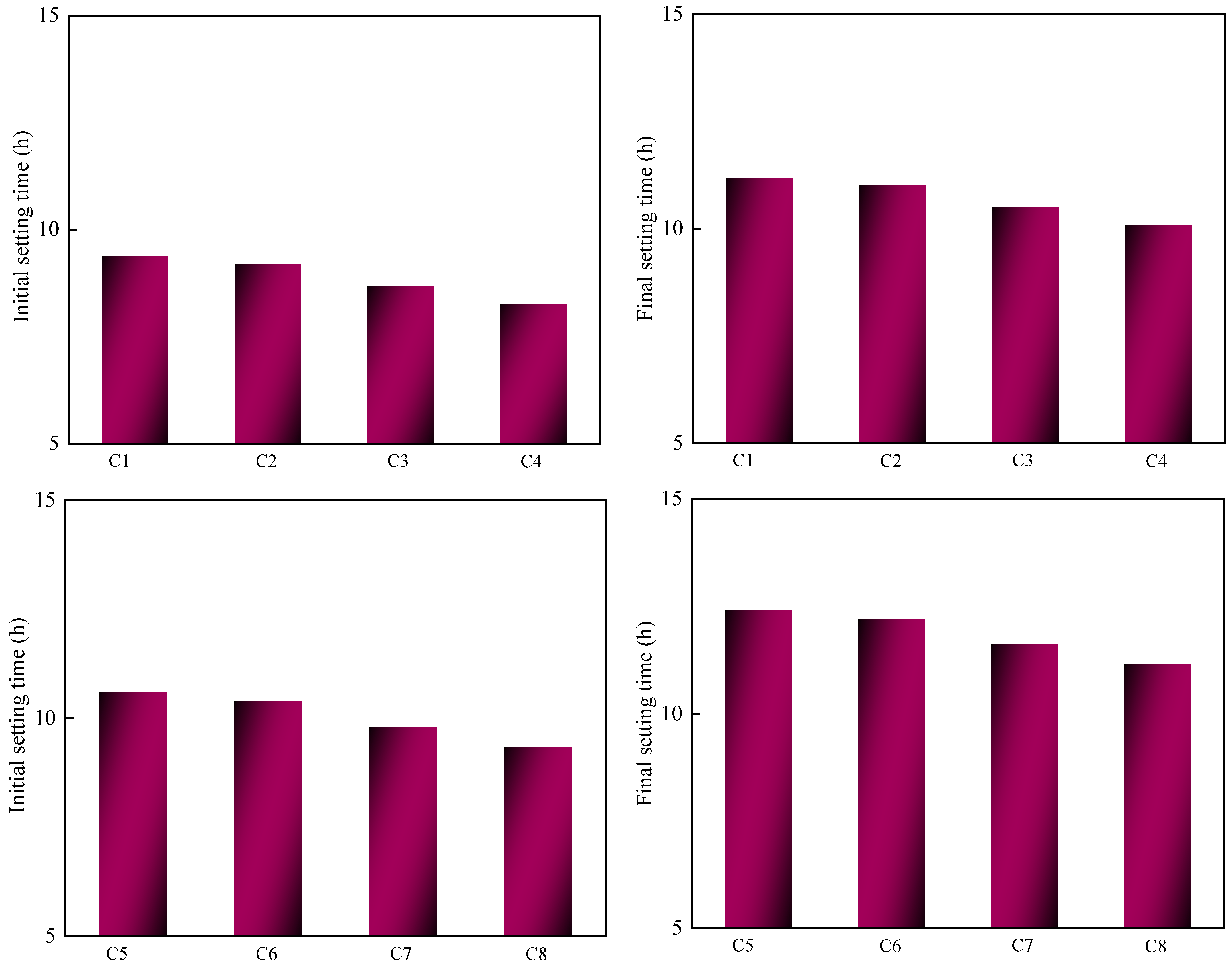








| Oxides | OPC | Loess |
|---|---|---|
| CaO | 65.544 | 11.663 |
| SiO2 | 18.691 | 58.335 |
| Al2O3 | 7.399 | 15.555 |
| MgO | 3.037 | 2.454 |
| Fe2O3 | 2.597 | 6.047 |
| Na2O | 0.296 | 1.211 |
| K2O | 0.576 | 2.887 |
| TiO2 | 0.604 | 1.101 |
| P2O5 | 0.089 | 0.187 |
| SO3 | 3.743 | 0.178 |
| MnO | 0.236 | 0.12 |
| SrO | 0.049 | 0.031 |
| Cl | 0.103 | 0.045 |
| ZrO2 | 0.024 | 0.05 |
| Sample Code | Mechanical Grinding of Loess (%) | Cement (%) | Water/Binder |
|---|---|---|---|
| G1 | 20 (G0) | 80 | 1.0 |
| G2 | 20 (G10) | 80 | 1.0 |
| G3 | 20 (G20) | 80 | 1.0 |
| G4 | 20 (G40) | 80 | 1.0 |
| G5 | 20 (G80) | 80 | 1.0 |
| G6 | 35 (G0) | 65 | 1.0 |
| G7 | 35 (G10) | 65 | 1.0 |
| G8 | 35 (G20) | 65 | 1.0 |
| G9 | 35 (G40) | 65 | 1.0 |
| G10 | 35 (G80) | 65 | 1.0 |
| G11 | 50 (G0) | 50 | 1.0 |
| G12 | 50(G10) | 50 | 1.0 |
| G13 | 50 (G20) | 50 | 1.0 |
| G14 | 50 (G40) | 50 | 1.0 |
| G15 | 50 (G80) | 50 | 1.0 |
| Sample Code | Single-Calcined Loess (%) | Cement (%) | Water/Binder |
| C1 | 20 (C400) | 80 | 1.0 |
| C2 | 20 (C550) | 80 | 1.0 |
| C3 | 20 (C700) | 80 | 1.0 |
| C4 | 20 (C850) | 80 | 1.0 |
| C5 | 35 (C400) | 65 | 1.0 |
| C6 | 35 (C550) | 65 | 1.0 |
| C7 | 35 (C700) | 65 | 1.0 |
| C8 | 35 (C850) | 65 | 1.0 |
| C9 | 50 (C400) | 50 | 1.0 |
| C10 | 50 (C550) | 50 | 1.0 |
| C11 | 50 (C700) | 50 | 1.0 |
| C12 | 50 (C850) | 50 | 1.0 |
| Parameterization | G0 | G10 | G20 | G40 | G80 |
|---|---|---|---|---|---|
| D10 (μm) | 15.89 | 2.24 | 1.78 | 1.59 | 1.42 |
| D30 (μm) | 35.57 | 10.02 | 7.96 | 7.10 | 4.48 |
| D50 (μm) | 50.24 | 22.44 | 17.83 | 20.00 | 12.62 |
| D60 (μm) | 56.37 | 28.25 | 25.18 | 28.25 | 17.83 |
| D90 (μm) | 112.47 | 63.25 | 56.37 | 70.96 | 44.77 |
| Cu = D60/D10 | 3.55 | 12.59 | 14.13 | 17.78 | 12.59 |
| Cc = D302/D60 × D10 | 1.41 | 1.58 | 1.41 | 1.12 | 0.79 |
| Ug = (D90 − D60)/D50 | 1.12 | 1.56 | 1.75 | 2.14 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Wang, K.; Zhang, X.; Jiang, Y.; Zhang, S. Potential Utilization of Loess in Grouting Materials: Effects of Grinding Time and Calcination Temperature. Minerals 2024, 14, 490. https://doi.org/10.3390/min14050490
Bai H, Wang K, Zhang X, Jiang Y, Zhang S. Potential Utilization of Loess in Grouting Materials: Effects of Grinding Time and Calcination Temperature. Minerals. 2024; 14(5):490. https://doi.org/10.3390/min14050490
Chicago/Turabian StyleBai, Hao, Kai Wang, Xiaoqiang Zhang, Yulong Jiang, and Shiyu Zhang. 2024. "Potential Utilization of Loess in Grouting Materials: Effects of Grinding Time and Calcination Temperature" Minerals 14, no. 5: 490. https://doi.org/10.3390/min14050490




