3.2.1. The Form of Impurity Elements in the High-Grade Matte
Table 4 lists the chemical phase analysis results of the high-grade matte. Evidently, As is mainly in the form of As
2S
3 and FeAsO
4. FeAsO
4 is easily decomposed into Fe
3O
4 As
2O
3 andCO
2 under converting conditions. There is also a small amount of As in the matte in the form of As
2O
3 and others. Sb mainly exists in the form of element Sb, Sb
2O
5 and Sb
2O
3 in the raw material, and a small part exists in the form of Sb
2S
3. Pb mainly exists in the form of PbS, PbO and element Pb in the raw materials, and a small part exists in the form of PbSiO
3.
Figure 5a shows the microscopic morphology of sample 1 (high-grade matte) in the converting process at 2000× magnification, and
Figure 5 shows the distribution of the elements around the impurity elements and the energy dispersive spectrometer analysis when the local area of the high-grade matte was amplified by 10,000× magnification. In the early stage of converting, the enrichment area of the impurity elements in the high-grade matte was relatively dispersed, the area was small, and the accumulation area of a few impurities was generally less than 3 μm. The analysis showed that most of the impurity elements in the high-grade matte were dispersed in the melt. The distribution of elements in the sample, combined with EDS analysis, showed that the main phase of the high-grade matte was Cu
2S. In the copper-poor region, it is a substance or compound of impurity elements, including Fe, As, Sb and Pb.
Table 4 lists that the content of FeAsO
4 in matte was 0.086%. Due to the low content, the FeAsO
4 phase may not be shown in
Figure 5. In addition, the FeAsO
4 may decompose into other phases. The impurities arsenic and antimony were enriched at the oxygen enrichment points, and their distribution in other areas was relatively dispersed, so the main phases of arsenic and antimony may be As
2O
3, Sb
2O
3, and small amounts of As
2S
3, Sb
2S
3, and Sb. The distribution of the impurities overlapped with that of sulfur, except for slight enrichment in the oxygen enrichment area. The main phases of lead are PbS and a small amount of PbO.
3.2.2. Impurity Element Content and Distribution in Melt
Table 5 and
Figure 6 show the variation in the contents of the As, Sb and Pb as the converting process of high-grade matte conversion progressed. Before 100 min of converting, the content of arsenic in the melt slowly decreased with the converting time, and after 130 min of converting, the content of arsenic gradually increased until it reached the highest value before copper production. It was found that the arsenic phase FeAsO
4 decompose and oxidized during the early stage of matte smelting, and some As
2O
3 volatilized into gas, while some As
2O
3 remained in the matte. As the sulfur in the melt was oxidized to SO
2, the overall melt mass decreased, and the content of arsenic increased.
Before converting oxygen for 100 min, the content of Sb in the melt slowly decreased with increasing converting time. It can be seen from the continuous oxygen conversion that the antimony content in the melt gradually increased. However, after 160 min of oxygen conversion, the antimony content in the melt decreased with an increasing oxygen conversion time. In the last 30 min, the Sb content increased from 0.051% to 0.056%. The analysis showed that the elemental antimony in the matte was oxidized to Sb2O3 and volatilized to gas, which decreased the content of antimony in the melt with increasing converting time. As the sulfur in the melt was oxidized to SO2, the overall mass of the melt decreased, and the content of antimony increased. The oxygen potential of the melt increased with the continuous blowing of oxygen-enriched air during the conversion process. The increased oxygen potential led to antimony to be oxidized to Sb2O3 into the gas or into the slag as Sb2O5. In addition, a large amount of SO2 was produced during this period, which provides favorable volatilization conditions for Sb2O3. Before the end of the converting process, the Sb content increased slightly due to the change in melt quality.
Because the lead phase in high-grade matte mainly includes PbO and Pb, some lead volatilizes into the gas during the slagging period. The quartz flux added in the middle of the converting process reacts with PbO in the melt to form PbSiO3, which makes the lead content in the melt change little. When Cu2O is present at the end of the converting process, PbS is oxidized to form volatile PbO, which enters the gas with a large amount of escaped SO2. The lead content in the blister copper was 0.24%, which seriously affected the subsequent smelting.
Figure 7a shows the microscopic morphology of sample 4 (white matte) in the converting process at 4000× magnification, and
Figure 7 shows the elemental distribution and energy dispersive spectrometer analysis of the impurities when the local area of the white matte was increased by 20,000× magnification. The main phase in the melt at the end of the slagging period of high-grade matte smelting was Cu
2S. The impurity elements began to gradually accumulate in the form of oxides or compounds, and the elements Fe, As and Sb exhibited a relatively obvious aggregation phenomenon. There were impurity particles measuring approximately 5 μm, which were wrapped in the Cu
2S phase. The distribution regions of the impurity elements As and Sb were consistent and overlapped with the oxygen-enriched regions, but the distribution of As was greatly affected by Si, while the element Sb was not. Therefore, arsenic may exist in the form of As
2O
3 or As
2O
5, and Sb may exist in the form of Sb
2O
3 or Sb
2O
5 or form complex compounds with other impurity elements. The distribution area of Pb in the white matte overlapped more with that of S and Cu and differed greatly from that of Si. It is speculated that Pb was not oxidized at the end of the slagging period and mainly existed in the Cu
2S phase in the form of PbS. The impurity particles in the melt in the middle stage of converting were centered on SiO
2, which was surrounded by oxides of Fe, As and Sb, while Pb was present in the Cu
2S phase in the form of PbS.
Figure 8a shows the microscopic morphology of sample 6 (blister copper) in the converting process at 1000× magnification, and
Figure 8 shows the distribution of elements around the impurity elements and energy dispersive spectrometer analysis when the local area of the blister copper was enlarged by 5000× magnification. The region denoted as point A is the Cu phase, point B is the matte phase, and point C is the impurity enrichment region. Before the end of the high-grade matte converting, the main phase of the product was element Cu, and the impurities As, Sb, and Pb and small amounts of Ni and Bi obviously aggregated. Most of the impurity particles were approximately 5 μm long, large particles with a radius of approximately 15 μm were also present, and impurity particles existed at the edge of the Cu
2S phase. The analysis revealed that the impurities gradually increased in abundance in the matte, and with the gradual transformation of Cu
2S to element Cu, the impurities gradually precipitated and increased in abundance. The distribution region of Pb overlapped with the distribution region of S and was not distributed in the Cu phase. It was presumed that Pb is not oxidized in matte at the end of copper converting and is not distributed in the Cu phase because PbS is oxidized after Cu
2S, which may mainly exist in the form of PbS in matte and impurity particles. The distributions of the impurities As and Sb overlapped with the distribution of oxygen, and it is speculated that As and Sb may exist in the form of oxides in blister copper.
Table 6 lists the element contents of converting slag. According to the matte, converting slag quality, the quality of arsenic entering the slag phase was 1.85% of the total. The distribution ratio of lead in slag was 12.21%. Because the total amount of antimony in matte is low, antimony could not be detected in the slag.
3.2.3. Phase Transformation Mechanism of Impurity Elements
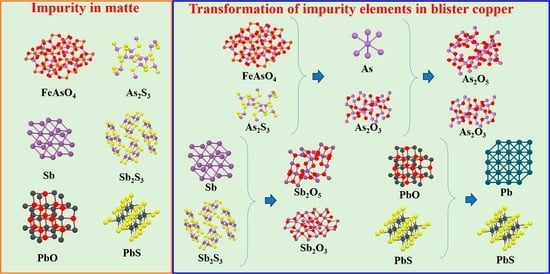
The phase transformation mechanism of the high-grade copper matte converting process into As is shown in
Figure 9. According to the thermodynamic equation for Reaction (3), As in the form of FeAsO
4 is decomposed into As
2O
3 during the process of converting. In addition, As in the form of element As and As
2S
3 can also be oxidized to As
2O
3 (Reactions (4) and (5)).
Comprehensive analysis showed that As
2O
3 can be partially volatilized to the gas phase, and some will remain in the melt. As
2O
3 in the melt is further oxidized to As
2O
5 (Reaction (6)) when the oxygen potential is high.
As
2O
5 is an acidic oxide that can combine with other basic oxides, such as CaO, to form a stable arsenate (Reaction (7)), which easily enters the slag phase.
The phase transformation mechanism of the high-grade copper matte conversion process into Sb is shown in
Figure 10. The antimony in the form of element Sb and Sb
2S
3 was oxidized to Sb
2O
3 (Reactions (8) and (9)) in the slagging period of converting, and Sb
2O
3 was volatile when heated.
Figure 10.
Migration and transformation of Sb during the process of conversion.
Figure 10.
Migration and transformation of Sb during the process of conversion.
Some unvolatilized Sb
2O
3 can be oxidized to Sb
2O
5 (Reaction (10)) in regions with high oxygen potential.
Sb
2O
5 is an acidic oxide that reacts with other basic oxides in the melt to form antimonate (Reaction (11)), which is stable, less dense, and easily enters the slag phase.
The phase transformation mechanism of the high-grade copper matte for converting Pb is shown in
Figure 11. Comprehensive analysis revealed that lead in the form of PbS is oxidized to PbO (Reaction (12)) at the end of copper production. Pb, PbS and PbO in the matte can be volatilized into gas under converting conditions.
PbO reacts with PbS to form Pb (Reaction (14)); the density of Pb is greater than that of Cu; and PbS easily remains in the blister copper to form impurities.
During the whole converting process, the unvolatilized PbO reacts with SiO
2 to form PbSiO
3 (Reaction (15)), which is the reason why the use of ferrosilicon slag in the converting process more easily removes lead.