Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Reagents
2.2. Cell Growth Inhibition Investigations by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.3. Apoptosis Detection Using A Histone/DNA ELISA
2.4. Soft Agar Colonization Assay
2.5. Culture of Normal Epithelial Cells with Copper Enrichment
2.6. Migration of Cells Assay
2.7. Reverse Transcriptase PCR in Real-Time
2.8. Transfection of Small Interfering RNAs (siRNAs)
2.9. Statistical Data Analysis
3. Results
3.1. Resveratrol Suppresses Proliferation and Induces Apoptosis in Prostate Cancer Cells
3.2. Cuprous Chelator Inhibits Resveratrol-Induced Antiproliferation and Apoptosis in Cancer Cells but Not Iron and Zinc Chelators
3.3. Resveratrol Restricts the Growth of Cancer Cells in A Clonogenic Assay
3.4. Resveratrol Induces Cell Death in Cancer Cells through ROS Production
3.5. The Antiproliferative Effect of Resveratrol Is Enhanced in Non-Tumorigenic Epithelial Cells When Copper Supplementation Is Administered
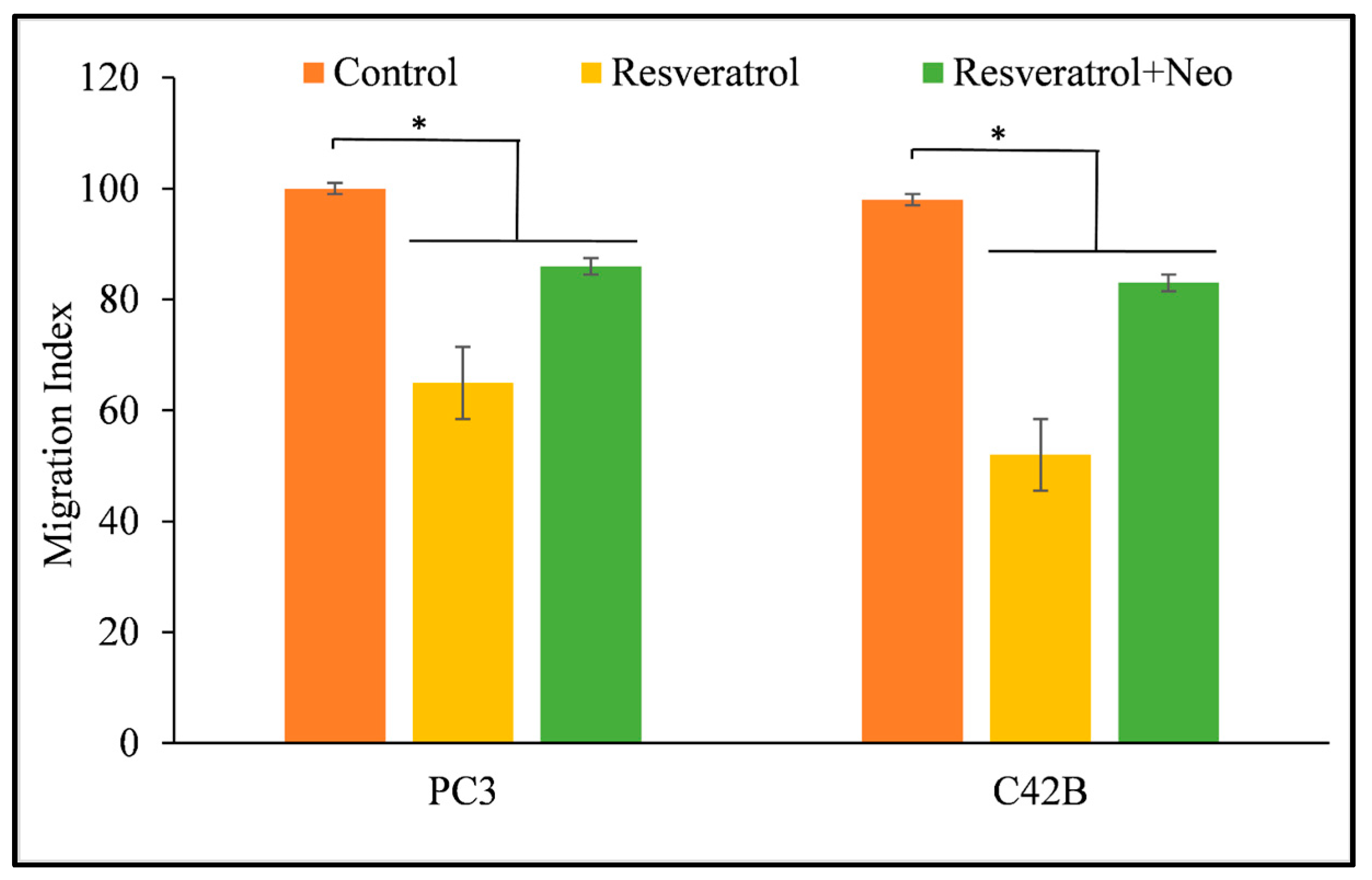
3.6. Copper Chelation Reverses the Migration of Cancer Cells That Is Inhibited by Resveratrol
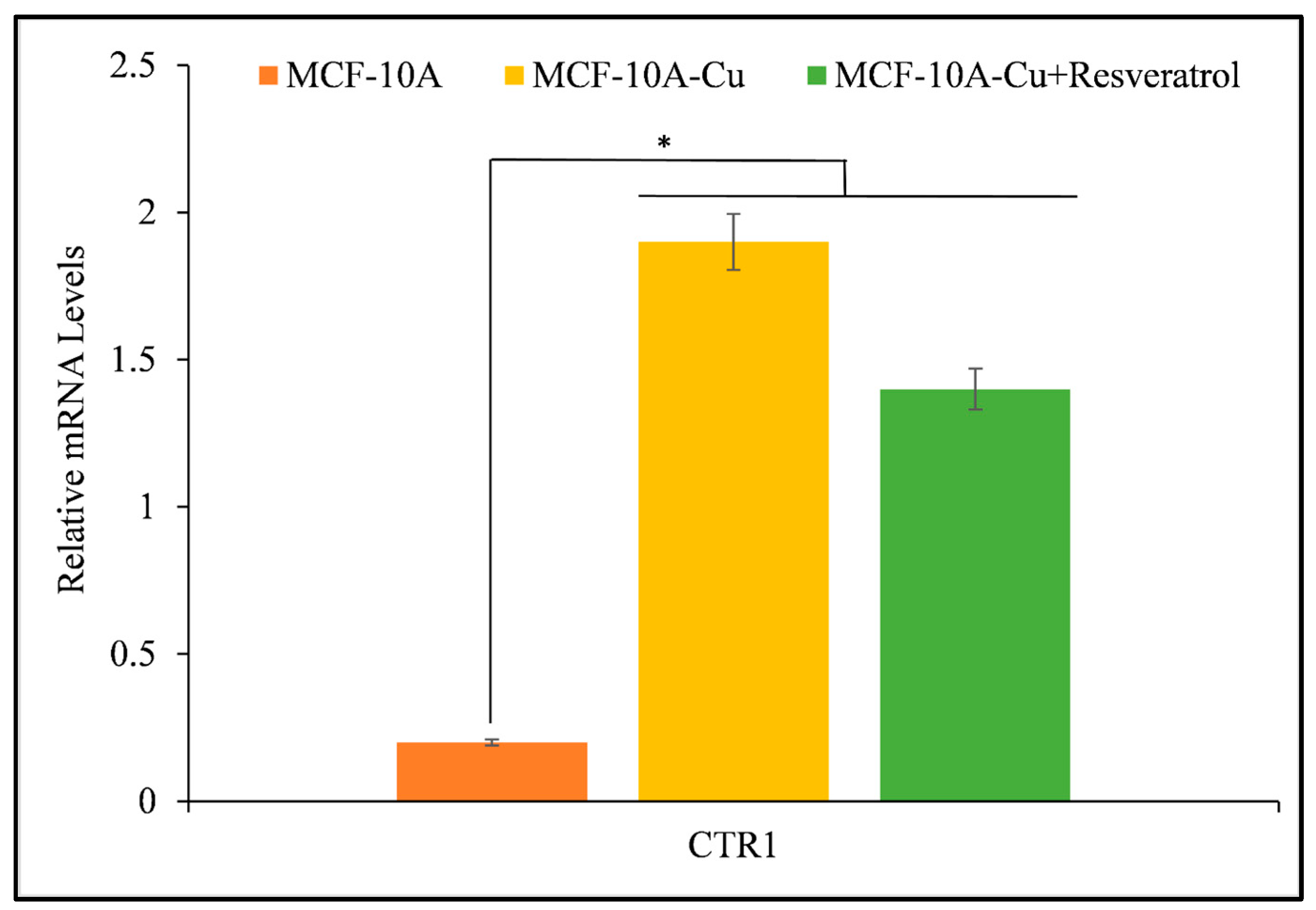
3.7. Resveratrol Inhibits the Activity of Copper Transporter CTR1
3.8. Resveratrol Minimizes Cell Proliferation in MCF-10A Cells Cultivated on Copper-Supplemented Media via Targeted Silencing of CTR1
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhan, M.; Rizvi, A. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S. The French paradox three decades later: Role of inflammation and thrombosis. Clin. Chim. Acta 2020, 510, 160–169. [Google Scholar] [CrossRef] [PubMed]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Michno, A.; Grużewska, K.; Ronowska, A.; Gul-Hinc, S.; Zyśk, M.; Jankowska-Kulawy, A. Resveratrol Inhibits Metabolism and Affects Blood Platelet Function in Type 2 Diabetes. Nutrients 2022, 14, 1633. [Google Scholar] [CrossRef]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13, 1934578X1801300923. [Google Scholar] [CrossRef]
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharm. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharm. 2018, 9, 1261. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Kinsella, J.E. Inhibition of Human LDL Oxidation by Resveratrol. Lancet 1993, 341, 1103–1104. [Google Scholar] [CrossRef]
- Belguendouz, L.; Fremont, L.; Linard, A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem. Pharmacol. 1997, 53, 1347–1355. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Zulueta, A.; Caretti, A.; Signorelli, P.; Ghidoni, R. Resveratrol: A potential challenger against gastric cancer. World J. Gastroenterol. 2015, 21, 10636–10643. [Google Scholar] [CrossRef]
- Aluyen, J.K.; Ton, Q.N.; Tran, T.; Yang, A.E.; Gottlieb, H.B.; Bellanger, R.A. Resveratrol: Potential as anticancer agent. J. Diet. Suppl. 2012, 9, 45–56. [Google Scholar] [CrossRef]
- Colin, D.; Limagne, E.; Jeanningros, S.; Jacquel, A.; Lizard, G.; Athias, A.; Gambert, P.; Hichami, A.; Latruffe, N.; Solary, E.; et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev. Res. 2011, 4, 1095–1106. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Resveratrol modulation of signal transduction in apoptosis and cell survival: A mini-review. Cancer Detect. Prev. 2006, 30, 217–223. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Davis, F.B.; Davis, P.J. Resveratrol and apoptosis. Ann. N. Y. Acad. Sci. 2011, 1215, 79–88. [Google Scholar] [CrossRef]
- Whitlock, N.C.; Baek, S.J. The anticancer effects of resveratrol: Modulation of transcription factors. Nutr. Cancer 2012, 64, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Rudek, M.A.; Zhou, X.C.; Wagner, W.D.; Hudson, T.S.; Anders, N.; Hammers, H.J.; Dowling, D.; King, S.; Antonarakis, E.S.; et al. A phase i study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 2015, 75, 1518–1525. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jorgensen, J.O.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, psa levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase i randomized, double-blind pilot study of micronized resveratrol (srt501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; Oirdi, M.E.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef]
- Arif, H.; Sohail, A.; Farhan, M.; Rehman, A.A.; Ahmad, A.; Hadi, S.M. Flavonoids-induced redox cycling of copper ions leads to generation of reactive oxygen species: A potential role in cancer chemoprevention. Int. J. Biol. Macromol. 2018, 106, 569–578. [Google Scholar] [CrossRef]
- Farhan, M.; Zafar, A.; Chibber, S.; Khan, H.Y.; Arif, H.; Hadi, S.M. Mobilization of copper ions in human peripheral lymphocytes by catechins leading to oxidative DNA breakage: A structure activity study. Arch. Biochem. Biophys. 2015, 580, 31–40. [Google Scholar] [CrossRef]
- Farhan, M.; Aatif, M.; Hadi, S.M.; Ahmad, A. Mechanism of Gallic Acid Anticancer Activity Through Copper-Mediated Cell Death. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychoudhury, S., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Fukuhara, K.; Miyata, N. Resveratrol as a new type of DNA-cleaving agent. Bioorganic Med. Chem. Lett. 1998, 8, 3187–3192. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the prooxidant action of plant polyphenols in the cellular microenvironment of malignant cells: Role of copper and therapeutic implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Rizvi, A.; Furkan, M.; Naseem, I. Physiological serum copper concentrations found in malignancies cause unfolding induced aggregation of human serum albumin in vitro. Arch. Biochem. Biophys. 2017, 636, 71–78. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Farhan, M. Naringin’s Prooxidant Effect on Tumor Cells: Copper’s Role and Therapeutic Implications. Pharmaceuticals 2022, 15, 1431. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Faisal, M.; Ullah, M.F.; Farhan, M.; Sarkar, F.H.; Ahmad, A.; Hadi, S.M. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: A mechanism for cancer chemopreventive action. Mol. Nutr. Food Res. 2014, 58, 437–446. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with Copper: From Copper Linked Mechanisms to Copper-Based Clinical Cancer Therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: Implications for a cancer preventive mechanism. Biometals 2011, 24, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.G.; Chen, D.; Orlu, S.; Cui, Q.C.; Miller, F.R.; Dou, Q.P. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Res. 2005, 7, R897–R908. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols Content, Antioxidant Activity, and Cytotoxicity Assessment of Taraxacum officinale Extracts Prepared through the Micelle-Mediated Extraction Method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Food Polyphenols as Preventive Medicine. Antioxidants 2023, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Pinto, B.; Costa, L.; Felgueira, E.; Fonseca, B.M.; Rebelo, I. Low Doses of Resveratrol Protect Human Granulosa Cells from Induced-Oxidative Stress. Antioxidants 2021, 10, 561. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, D.; Zhou, S.; Dong, N.; Ji, Y.; An, P.; Wang, J.; Luo, Y.; Luo, J. Regulatory roles of copper metabolism and cuproptosis in human cancers. Front. Oncol. 2023, 13, 1123420. [Google Scholar] [CrossRef]
- Bian, C.; Zheng, Z.; Su, J.; Chang, S.; Yu, H.; Bao, J.; Xin, Y.; Jiang, X. Copper homeostasis and cuproptosis in tumor pathogenesis and therapeutic strategies. Front. Pharmacol. 2023, 14, 1271613. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer Therapy by Catechins Involves Redox Cycling of Copper Ions and Generation of Reactive Oxygen Species. Toxins 2016, 8, 37. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef]
- Bhadra, K. A Mini Review on Molecules Inducing Caspase-Independent Cell Death: A New Route to Cancer Therapy. Molecules 2022, 27, 6401. [Google Scholar] [CrossRef]
- Leist, M.; Jäättelä, M. Four deaths and a funeral: From caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2001, 2, 589–598. [Google Scholar] [CrossRef]
- Mu, Q.; Najafi, M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int. Immunopharmacol. 2021, 98, 107895. [Google Scholar] [CrossRef]
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef]
- Varesi, A.; Chirumbolo, S.; Campagnoli, L.I.M.; Pierella, E.; Piccini, G.B.; Carrara, A.; Ricevuti, G.; Scassellati, C.; Bonvicini, C.; Pascale, A. The Role of Antioxidants in the Interplay between Oxidative Stress and Senescence. Antioxidants 2022, 11, 1224. [Google Scholar] [CrossRef]
- Watson, J. Oxidants, Antioxidants and the Current Incurability of Metastatic Cancers. Open Biol. 2013, 3, 120144. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 4562. [Google Scholar] [CrossRef] [PubMed]








| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| CTR1 | 5′- GCT GGA AGA AGG CAG TGG TA-3′ | 5′- AAA GAG GAG CAA GAA GGG ATG -3′ |
| GADPH | 5′- TGG GTG TGA ACC ATG AGA AGT -3′ | 5′- TGA GTC CTT CCA CGA TAC CAA -3′ |
| Cell Line | Treatment | Apoptosis (Folds) | Inhibition of Apoptosis (%) |
|---|---|---|---|
| PC3 | Untreated | - | - |
| Resveratrol | 2.32 ± 0.2 | - | |
| +TU | 1.11 ± 0.2 | 52.15 | |
| +SOD | 1.27 ± 0.1 | 45.25 | |
| +CAT | 1.38 ± 0.1 | 40.15 | |
| C42B | Untreated | - | - |
| Resveratrol | 2.41 ± 0.3 | - | |
| +TU | 1.05 ± 0.1 | 45.22 | |
| +SOD | 1.18 ± 0.2 | 51.03 | |
| +CAT | 1.23 ± 0.1 | 48.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life 2024, 14, 611. https://doi.org/10.3390/life14050611
Farhan M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life. 2024; 14(5):611. https://doi.org/10.3390/life14050611
Chicago/Turabian StyleFarhan, Mohd. 2024. "Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species" Life 14, no. 5: 611. https://doi.org/10.3390/life14050611






