Inflammatory Biomarkers for Assessing In-Hospital Mortality Risk in Severe COVID-19—A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
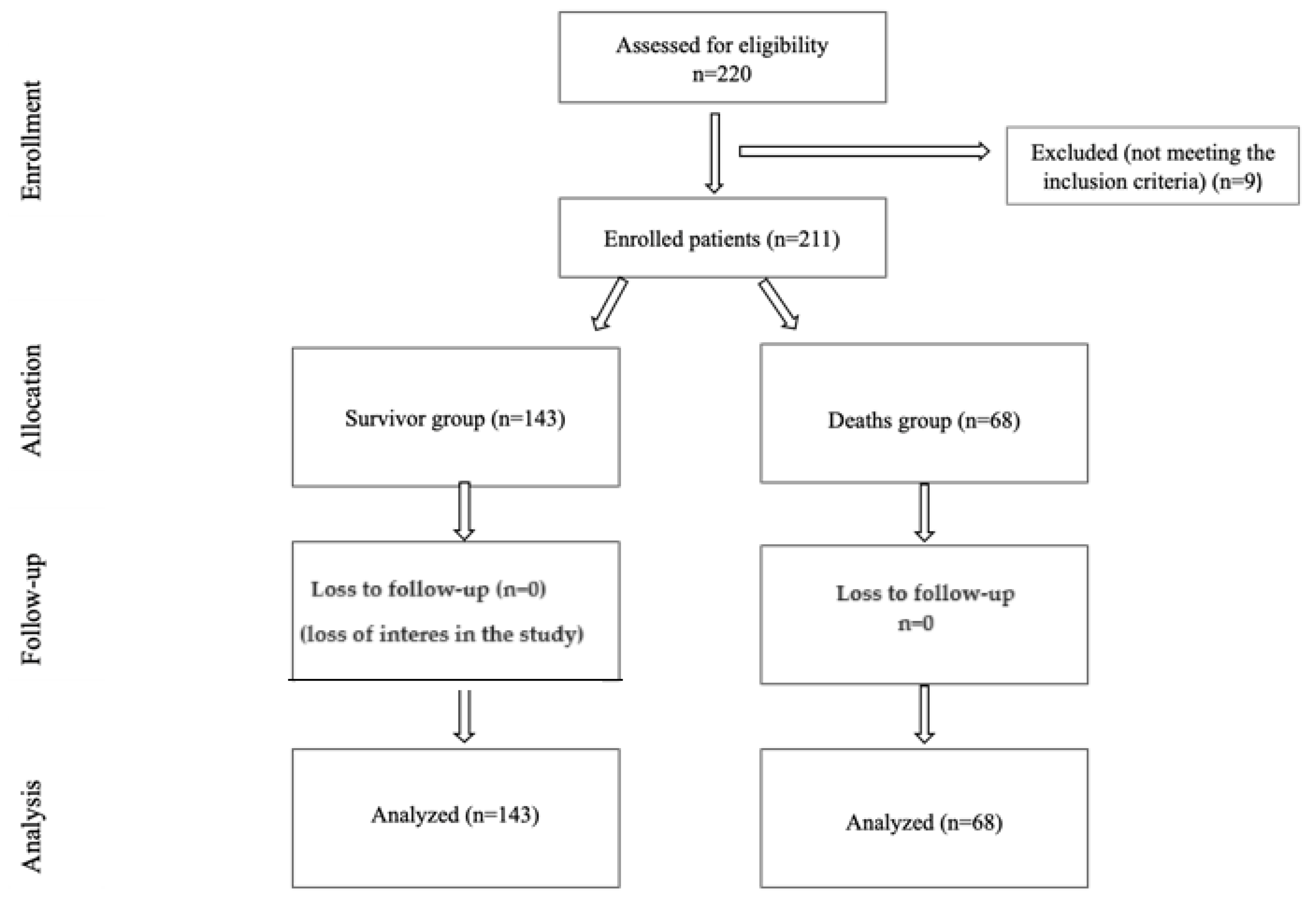
2.2. Study Participants and Inclusion and Exclusion Criteria
2.3. Data Collection
- ✓
- An automatic analyzer that employs flow cytometry with fluorescence, utilizing a semiconductor LASER and hydrodynamic focusing for blood count and leukocyte formula determination.
- ✓
- A latex-enhanced immunoturbidimetric method for assessing CRP and procalcitonin levels.
2.4. Statistical Analysis
3. Results
Characteristics of the Population
- For age, the AUC is 0.7157 with a p-value of less than 0.0001. The recommended threshold is over 60 years, with a Youden J index of 0.3579. This threshold provides a sensitivity of 88.24% and specificity of 47.55% for predicting in-hospital death.
- For the granulocyte-to-lymphocyte ratio on the 14th day of hospitalization (4th day of ICU admission), the AUC is 0.7789 with a p-value of less than 0.0001. The recommended threshold is over 10, with a Youden J index of 0.4528. This threshold offers a sensitivity of 69.64% and specificity of 74.19% for predicting in-hospital death.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health. 2020, 13, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi-Kangevari, M.; Abbastabar, H.; ElHafeez, S.A.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations,1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 18. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Dong, H.; Xia, S.Q.; Huang, Y.Z.; Wang, D.; Zhao, Y.; Liu, W.-H.; Tu, S.-H.; Zhang, M.-M.; Wang, Q.; et al. Correlation Analysis Between Disease Severity and Inflammation-related Parameters in Patients With COVID-19 Pneumonia. BMC Infect. Dis. 2020, 20, 963. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Alkhatip, A.A.A.M.M.; Kamel, M.G.; Hamza, M.K.; Farag, E.M.; Yassin, H.M.; Elayashy, M.; Naguib, A.A.; Wagih, M.; Abd-Elhay, F.A.-E.; Algameel, H.Z.; et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: A systematic review and meta-analysis. Expert Rev. Mol. Diagn. 2021, 21, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Cuihong, X.; Ke, M.; Ke, S.; Wei, W.; et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 72, 762–768. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 1–3. [Google Scholar]
- Elliot, J.G.; Donovan, G.M.; Wang, K.; Green, F.; James, A.L.; Noble, P.B. Fatty airways: Implications for obstructive disease. Eur. Respir. J. 2019, 54, 1900857. [Google Scholar] [CrossRef] [PubMed]
- Földi, M.; Farkas, N.; Kiss, S.; Dembrovszky, F.; Szakács, Z.; Balaskó, M.; Erőss, B.; Hegyi, P.; Szentesi, A. Visceral adiposity elevates the risk of critical condition in COVID-19: A systematic review and meta-analysis. Obesity 2020, 29, 521–528. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, B.; Liang, S.; Yang, J.-W.; Lu, H.-W.; Chai, Y.-H.; Wang, L.; Zhang, L.; Li, Q.-H.; Zhao, L.; et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur. Respir. J. 2020, 55, 2001112. [Google Scholar] [CrossRef]
- Wafa, M.H.; Stanikzai, M.H.; Fazli, N. Biopsychosocial Profile of COVID-19 Patients Cared for in Public and Privat Health Facilities in Kandahar Province. Afganistan. Ment. Illn. 2023, 2023, 2669168. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cao, Y.Y.; Tan, G.; Dong, X.; Wang, B.C.; Lin, J.; Yan, Y.Q.; Liu, G.H.; Akdis, M.; Akdis, C.A.; et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021, 76, 533–550. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic Human Coronavirus Infections: Causes and Consequences of Cytokine Storm and Immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Song, J.W.; Zhang, C.; Fan, X.; Meng, F.P.; Xu, Z.; Xia, P.; Cao, W.J.; Yang, T.; Dai, X.P.; Wang, S.Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 3410. [Google Scholar] [CrossRef] [PubMed]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Giannella, M.; Scudeller, L.; Tedeschi, S.; Rinaldi, M.; Bussini, L.; Fornaro, G.; Pascale, R.; Pancaldi, L.; Pasquini, Z.; et al. Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: A multicentre cohort study (PREDI-CO study). Clin. Microbiol. Infect. 2020, 26, 1545–1553. [Google Scholar] [CrossRef]
- Lockhart, S.M.; O’Rahilly, S. When two pandemics meet: Why is obesity associated with increased COVID-19 mortality? Med 2020, 1, 33–42. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Achamallah, N.; Ji, H.; Claggett, B.L.; Sun, N.; Botting, P.; Nguyen, T.-T.; Luong, E.; Kim, E.H.; Park, E.; et al. Pre-existing traits associated with Covid-19 illness severity. PLoS ONE 2020, 15, e0236240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.M.; Zhao, C.Y.; Tarquinio, K.M.; Brown, S.P. Causes and Consequences of COVID-19-Associated Bacterial Infections. Front. Microbiol. 2021, 12, 682571. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, Y.; Bai, T.; Xie, Y.; Huang, J.; Li, J.; Xiong, W.; Yang, D.; Chen, R.; Lu, F.; et al. COVID-19 with Different Severities: A Multicenter Study of Clinical Features. Am. J. Respir. Crit. Care Med. 2020, 201, 1380–1388. [Google Scholar] [CrossRef]
- Metzger, D.W.; Sun, K. Immune dysfunction and bacterial coinfections following influenza. J. Immunol. 2013, 191, 2047–2052. [Google Scholar] [CrossRef]
- McCullers, J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 2014, 12, 252–262. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Dela Vega, M.C.M.; Laviano, A. High Neutrophil to Lymphocyte Ratio as a Prognostic Marker in COVID-19 Patients. Clin. Nutr. ESPEN 2020, 40, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers Associated with COVID-19 Disease Progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Simadibrata, D.M.; Lubis, A.M. D-Dimer Levels on Admission and All-Cause Mortality Risk in COVID-19 Patients: A Meta-Analysis. Epidemiol. Infect. 2020, 148, e202. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, J.; Huang, X.; Liu, Y.; Yuan, Y.; Zhang, X.; Zou, C.; Xiao, K.; Wang, J. Prognostic Role of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio in Prostate Cancer: A Meta-Analysis of Results from Multivariate Analysis. Int. J. Surg. 2018, 60, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Lusczek, E.R.; Ingraham, N.E.; Karam, B.S.; Proper, J.; Siegel, L.; Helgeson, E.S.; Lotfi-Emran, S.; Zolfaghari, E.J.; Jones, E.; Usher, M.G.; et al. Characterizing COVID-19 clinical phenotypes and associated comorbidities and complication profiles. PLoS ONE 2021, 16, e0248956. [Google Scholar] [CrossRef] [PubMed]
- Brojakowska, A.; Eskandari, A.; Bisserier, M.; Bander, J.; Garikipati, V.N.; Hadri, L.; Goukassian, D.A.; Fish, K.M. Comorbidities, sequelae, blood biomarkers and their associated clinical outcomes in the Mount Sinai Health System COVID-19 patients. PLoS ONE 2021, 16, e0253660. [Google Scholar] [CrossRef] [PubMed]
- Pál, K.; Molnar, A.A.; Huțanu, A.; Szederjesi, J.; Branea, I.; Timár, Á.; Dobreanu, M. Inflammatory Biomarkers Associated with In-Hospital Mortality in Critical COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 10423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, W.H.; Tipih, T.; Makoah, N.A.; Vermeulen, J.G.; Goedhals, D.; Sempa, J.B.; Burt, F.J.; Taylor, A.; Mahalingam, S. Comorbidities in SARS-CoV-2 patients: A systematic review and meta-analysis. mBio 2021, 12, e03647-20. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Puri, A.; Wang, T.; Guo, S. Clinical features, comorbidities, complications and treatment options in severe and non-severe COVID-19 patients: A systemic review and meta-analysis. Nurs. Open 2021, 8, 1077–1088. [Google Scholar] [CrossRef]
- Callender, L.A.; Curran, M.; Bates, S.M.; Mairesse, M.; Weigandt, J.; Betts, C.J. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front. Immunol. 2020, 11, 1991. [Google Scholar] [CrossRef]
- Ntalouka, M.P.; Brotis, A.; Mermiri, M.; Pagonis, A.; Chatzis, A.; Bareka, M.; Kotsi, P.; Pantazopoulos, I.; Gourgoulianis, K.; Arnaoutoglou, E.M. Predicting the Outcome of Patients with Severe COVID-19 with Simple Inflammatory Biomarkers: The Utility of Novel Combined Scores-Results from a European Tertiary/Referral Centre. J. Clin. Med. 2024, 13, 967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Survivors (n = 143) | Deaths (n = 68) | Statistical Significance (p) | |
|---|---|---|---|
| Gender (M/F) | 62/81 | 32/36 | 0.7207 * |
| Age (years)—median (IQR) | 62 (48–71) | 72 (64–77) | <0.0001 ** |
| Environment of origin (U/R) | 113/30 | 44/24 | 0.0396 * |
| History of COPD—no. of patients (%) | 5 (3.5%) | 3 (4.4%) | 0.9519 * |
| History de DM—no. of patients (%) | 29 (20.3%) | 24 (35.3%) | 0.0292 * |
| History of depression—no. of patients (%) | 3 (2.1%) | 2 (2.9%) | 0.9141 * |
| Obesity—no. of patients (%) | 21 (14.7%) | 32 (47.1%) | <0.0001 * |
| Biochemical Parameters at the Time of Admission to ICU | Survivors (n = 143) | Deaths (n = 68) | Statistical Significance (p) |
|---|---|---|---|
| Leukocytes (×103/mm3)—median (IQR) | 6.58 (4.7–8.9) | 10.74 (6.3–15.3) | <0.0001 * |
| Neutrophiles (×103/mm3)—median (IQR) | 4.84 (3.0–7.3) | 9.29 (5.9–13.6) | <0.0001 * |
| Lymphocytes (×103/mm3)—median (IQR) | 0.91 (0.6–1.4) | 0.61 (0.4–0.9) | <0.0001 * |
| Granulocytes/Lymphocytes ratio—median (IQR) | 4.77 (2.6–9.4) | 14.74 (7.0–28.3) | <0.0001 * |
| Procalcitonin (ng/mL): | |||
| <0.5 | 129 (90.2%) | 46 (67.6%) | 0.0002 ** |
| 0.5–2 | 13 (9.1%) | 16 (23.5%) | |
| 2–10 | 1 (0.7%) | 4 (5.9%) | |
| >10 | 0 (0.0%) | 2 (2.9%) | |
| CRP (mg/L)—median (IQR) | 44.35 (11.2–96.5) | 96.46 (37.0–181.4) | 0.0001 * |
| Ferritin (ng/mL)—median (IQR) | 655 (272–1194) | 1128.3 (619.3–2085.3) | 0.0001 * |
| Biochemical Parameters on the 14th Day of Hospitalization | Survivors (n = 143) | Deaths (n = 68) | Statistical Significance (p) |
|---|---|---|---|
| Leukocytes (×103/mm3)—median (IQR) | 8.38 (6.3–11.1) | 9.29 (7.4–15.1) | 0.0169 * |
| Neutrophiles (×103/mm3)—median (IQR) | 6.54 (4.4–9.4) | 8.40 (6.5–13.4) | 0.0004 * |
| Lymphocytes (×103/mm3)—median (IQR) | 1.08 (0.7–1.6) | 0.58 (0.3–0.9) | <0.0001 * |
| Granulocytes/Lymphocytes ratio—median (IQR) | 6.41 (2.9–10.4) | 13.35 (8.5–25.9) | <0.0001 * |
| Procalcitonin (ng/mL): | |||
| <0.5 | 115 (80.4%) | 37 (54.4%) | 0.0010 ** |
| 0.5–2 | 5 (3.5%) | 6 (8.8%) | |
| 2–10 | 1 (0.7%) | 5 (7.4%) | |
| >10 | 0 (0.0%) | 1 (1.5%) | |
| CRP (mg/L)—median (IQR) | 11.54 (4–40) | 41.28 (17.5–98) | <0.0001 * |
| Ferritin (ng/mL)—median (IQR) | 647.4 (318.6–1123.5) | 1197.1 (683.5–2397.8) | <0.0001 * |
| Survivors (n = 143) | Deaths (n = 68) | Statistical Significance (p) | |
|---|---|---|---|
| Treatment with convalescent plasma—no. of patients (%) | 3 (2.1%) | 5 (7.4%) | 0.1383 * |
| Treatment with immunomodulator (Tocilizumab)—no. of patients (%) | 7 (4.9%) | 4 (5.9%) | 0.9762 * |
| Number of days in ICU—median (IQR) | 12 (8–14) | 13 (7–17.5) | 0.2196 ** |
| Relative Risk | Confidence Interval 95% | |
|---|---|---|
| Age | 1.0749 | 1.0337—1.1178 |
| The presence of obesity at ICU admission | 6.0525 | 2.3121 to 15.8439 |
| Procalcitonin > 10 ng/mL at ICU admission | 4.23 × 106 | |
| G/L ratio on the 4th day of ICU | 1.0922 | 1.0097 to 1.1814 |
| Procalcitonin > 10 ng/mL at 4th day in ICU | 4.02087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bimbo-Szuhai, E.; Botea, M.O.; Romanescu, D.D.; Beiusanu, C.; Gavrilas, G.M.; Popa, G.M.; Antal, D.; Bontea, M.G.; Sachelarie, L.; Macovei, I.C. Inflammatory Biomarkers for Assessing In-Hospital Mortality Risk in Severe COVID-19—A Retrospective Study. J. Pers. Med. 2024, 14, 503. https://doi.org/10.3390/jpm14050503
Bimbo-Szuhai E, Botea MO, Romanescu DD, Beiusanu C, Gavrilas GM, Popa GM, Antal D, Bontea MG, Sachelarie L, Macovei IC. Inflammatory Biomarkers for Assessing In-Hospital Mortality Risk in Severe COVID-19—A Retrospective Study. Journal of Personalized Medicine. 2024; 14(5):503. https://doi.org/10.3390/jpm14050503
Chicago/Turabian StyleBimbo-Szuhai, Erika, Mihai Octavian Botea, Dana Diana Romanescu, Corina Beiusanu, Gabriela Maria Gavrilas, Georgiana Maria Popa, Dania Antal, Mihaela Gabriela Bontea, Liliana Sachelarie, and Iulia Codruta Macovei. 2024. "Inflammatory Biomarkers for Assessing In-Hospital Mortality Risk in Severe COVID-19—A Retrospective Study" Journal of Personalized Medicine 14, no. 5: 503. https://doi.org/10.3390/jpm14050503






