Fast Bacterial Succession Associated with the Decomposition of Larix gmelinii Litter in Wudalianchi Volcano
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design and Sample Analysis
2.3. Sample Collection
2.4. Measurement
2.5. Statistical Analysis
3. Results
3.1. Shifts in Nutrient Elements with the Decomposition of Plant Litter
3.2. Shifts in Bacterial Community with the Decomposition of Plant Litter
3.3. Microbial Diversity Changes during Litter Decomposition
3.4. β-Diversity
3.5. RDA Analysis
3.6. Bacterial Function Prediction in Litter
4. Discussion
4.1. Variation in Nutrient Elements before and after Litter Decomposition
4.2. Differences in Bacterial Community Structure and Diversity in Litter
4.3. Relationship between Nutrient Elements and Bacterial Community Structure in Litters
4.4. Potential Functional Groups of Soil Microbes in Litter Decomposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, Z.X.; Zhu, B.B.; Tian, Q.X.; Lin, Q.L.; Chen, L. Advances in the study of home field dominance in leaf apoplastic decomposition. Chin. J. Plant Ecol. 2023, 47, 597–607. [Google Scholar]
- Polyakova, O.; Billor, N. Impact of deciduous tree species on litterfall quality, decomposition rates and nutrient circulation in pine stands. For. Ecol. Manag. 2007, 253, 11–18. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.J.; Xu, C.; Liu, X.F.; Xiong, D.C.; Lin, C.F. Effects of forest conversion on nutrient return and nutrient use efficiency of apoplastic litter in the Central Subtropics. Chin. J. Appl. Ecol. 2022, 33, 321–328. [Google Scholar]
- Zeng, Q.C.; Liu, Y.; Zhang, H.X.; An, S.S. Fast bacterial succession associated with the decomposition of Quercus wutaishanica litter on the Loess Plateau. Biogeochemistry 2019, 144, 119–131. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.P.; Zhao, C.Y.; Yu, P.Y.; Jia, H.T. Rapid microbial community evolution in initial Carex litter decomposition stages in Bayinbuluk alpine wetland during the freeze–thaw period. Ecol. Indic. 2021, 121, 107180–107192. [Google Scholar] [CrossRef]
- Lu, Y.; Li, K.; Liang, Q.; Li, C.R.; Zhang, C.H. Effects of leaf apoplastic decomposition of four dominant afforestation species in Taishan on the structure of bacterial communities within apoplasts. Acta Ecol. Sin. 2019, 39, 3175–3186. [Google Scholar]
- Strickland, M.S.; Osburn, E.; Lauber, C.; Fierer, N.; Bradford, M.A. Litter quality is in the eye of the beholder: Initial decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009, 23, 627–636. [Google Scholar] [CrossRef]
- Gołębiewski, M.; Tarasek, A.; Sikora, M.; Deja-Sikora, E.; Tretyn, A.; Niklińska, M. Rapid microbial community changes during initial stages of pine litter decomposition. Microb. Ecol. 2018, 77, 56–75. [Google Scholar] [CrossRef]
- Romaní, A.M.; Fischer, H.; Tranvik, M.L.J. Interactions of bacteria and fungi on decomposing litter: Differential extracellular enzyme activities. Ecology 2006, 87, 2559–2569. [Google Scholar] [CrossRef]
- Dong, X.D.; Gao, P.; Zhou, R.; Li, C.; Dun, X.J.; Niu, X. Changing characteristics and influencing factors of the soil microbial community during litter decomposition in a mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. forest in Northern China. Catena 2021, 196, 104811–104822. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yin, X.Q.; Kou, X.C.; Wang, Z.H.; Li, X.Q.; Jiang, Y.F.; Wang, H.X.; Bernard, E.C. Effects of soil fauna on cellulose and lignin decomposition of plant litter in the Changbai Mountain, China. Environ. Entomol. 2019, 48, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Sci. 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Kohl, L.; Myers-Pigg, A.; Edwards, K.; Billings, S.; Warren, J.; Podrebarac, F.; Ziegler, S. Microbial inputs at the litter layer translate climate into altered organic matter properties. Glob. Chang. Biol. 2020, 27, 435–453. [Google Scholar] [CrossRef]
- Certini, G.; Kwon, T.; Rompato, B.; Djukic, I.; Forte, C. Decomposition of green tea and rooibos tea across three monospecific temperate forests: Effect of litter type and tree species. Heliyon 2023, 9, e16689. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Berg, B. Import and release of nutrients during the first five years of plant litter decomposition. Soil Biol. Biochem. 2023, 176, 108878. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Otaki, M.; Tsuyuzaki, S. Succession of litter-decomposing microbial organisms in deciduous birch and oak forests, northern Japan. Acta Oecol. 2019, 101, 103485. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Xie, L.H.; Cao, H.J.; Wang, L.M.; Yang, F.; Wang, J.F.; Liu, Y.N.; Ni, H.W. Effects of bacteria on the early decomposition of apoplastic material in Wudalianchi volcanic forests. Chin. J. Appl. Ecol. 2023, 34, 1941–1948. [Google Scholar]
- Deligne, N.I.; Cashman, K.V.; Roering, J.J. After the lava flow: The importance of external soil sources for plant colonization of recent lava flows in the central Oregon Cascades, USA. Geomorphology 2013, 202, 15–32. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Xu, L.J.; Zhang, Y.H.; Xia, C.M.; Li, H.G.; Liu, T. An analysis of the ecological value of Wudalianchi, Heilongjiang Province, China. Biodiver. Sci. 2011, 19, 63–70. [Google Scholar]
- Huang, Q.Y.; Yang, F.; Xie, L.H.; Cao, H.J.; Luo, C.Y.; Wang, J.F.; Yan, Z.Y.; Ni, H.W. Diversity and communlty structure of soil bacteria in different volcanoes, Wudalianchi. Acta Ecol. Sin. 2021, 41, 8276–8284. [Google Scholar]
- Huang, Q.Y.; Cao, H.J.; Wang, L.M.; Xie, L.H.; Ni, H.W. Species diversity and soil nutrients in lava platforms of Wudalianchi. J. Z. Agric. For. Univ. 2019, 36, 80–87. [Google Scholar]
- Wright, M.S.; Covich, A.P. Relative importance of bacteria and fungi in a tropical headwater stream: Leaf decomposition and invertebrate feeding preference. Microb. Ecol. 2005, 49, 536–546. [Google Scholar] [CrossRef]
- Jia, T.; Liang, X.X.; Guo, T.Y.; Wu, T.H.; Chai, B.F. Bacterial community succession and influencing factors for Imperata cylindrica litter decomposition in a copper tailings area of China. Sci. Total Environ. 2022, 815, 152908. [Google Scholar] [CrossRef]
- Estrada-De Los Santos, P.; Bustillos-Cristales, R.; Caballero-Mellado, J. Burkholderia, a Genus Rich in Plant-Associated Nitrogen Fixers with Wide Environmental and Geographic Distribution. Appl. Environ. Microbiol. 2001, 67, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
- Huang, Q.Y.; Xie, L.Y.; Cao, H.Y.; Yang, F.; Ni, H.W. Variation characteristics of leaf functional traits of Populus davidiana in Wudalianchi Volcano, northeastern China. J. Beijing For. Univ. 2021, 43, 81–89. [Google Scholar]
- Zhu, Y.G.; Peng, J.J.; Chen, C.; Xiong, C.; Li, S.L.; Ge, A.H.; Wang, E.T.; Liesack, W. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023, 28, 1391–1405. [Google Scholar] [CrossRef]
- Daebeler, A.; Petrová, E.; Kinz, E.; Grausenburger, S.; Berthold, H.; Sandén, T.; Angel, R. Pairing litter decomposition with microbial community structures using the Tea Bag Index (TBI). Soil 2021, 8, 163–176. [Google Scholar] [CrossRef]
- Liber, J.A.; Minier, D.H.; Stouffer-Hopkins, A.; Van Wyk, J.; Longley, R.; Bonito, G. Maple and hickory leaf litter fungal communities reflect pre-senescent leaf communities. PeerJ 2022, 10, e12701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Huang, T.; Zhao, M.L.; Hu, Z.H.; Ni, Y.; Jiang, J.Q.; Cheng, B.J.; Li, X.Y.; Chen, J. Comparison of soil microbial abundances and co-occurrence networks in the volcanic soil of the cone and crater. Catena 2024, 236, 107734. [Google Scholar] [CrossRef]
- Tláskal, V.; Voříšková, J.; Baldrian, P. Bacterial succession on decomposing leaf litter exhibits a specific occurrence pattern of cellulolytic taxa and potential decomposers of fungal mycelia. FEMS Microbiol. Ecol. 2016, 92, fiw177. [Google Scholar] [CrossRef]
- Baldani, J.I.; Reis, V.M.; Videira, S.S.; Boddey, L.H.; Baldani, V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: A practical guide for microbiologists. Plant Soil. 2014, 384, 413–431. [Google Scholar] [CrossRef]
- Aislabie, J.; Broady, P.A.; Saul, D.J. Culturable aerobic heterotrophic bacteria from high altitude, high latitude soil of La Gorce Mountains (86°30′ S, 147° W). Antarct. Sci. 2006, 18, 313–321. [Google Scholar] [CrossRef]
- Liang, X.L.; Wang, Y.W.; Chai, B.F.; Jia, T. Study on microbial community function of Bothriochloa ischaemum litter at different decomposition stages in copper tailings area. J. Shanxi Univ. 2024, 47, 227–237. [Google Scholar]
- Li, F.; Hu, J.Y.; Xie, Y.H.; Yang, G.S.; Hu, C.; Chen, X.S.; Deng, Z.M. Foliar stoichiometry of carbon, nitrogen, and phosphorus in wetland sedge Carex brevicuspis along a small-scale elevation gradient. Ecol. Indic. 2018, 92, 322–329. [Google Scholar] [CrossRef]
- Purahong, W.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Däumlich, V.; Mital, S.; Buscot, F.; Hofrichter, M.; Gutknecht, J.L.M.; Krüger, D. Uncoupling of microbial community structure and function in decomposing litter across beech forest ecosystems in Central Europe. Sci. Rep. 2014, 4, 7014. [Google Scholar] [CrossRef]
- Yarwood, S.A. The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: A critical review. FEMS Microbiol. Ecol. 2018, 94, fiy175. [Google Scholar] [CrossRef]
- Purahong, W.; Kapturska, D.; Pecyna, M.J.; Jariyavidyanont, K.; Kaunzner, J.; Juncheed, K.; Uengwetwanit, T.; Rudloff, R.; Schulz, E.; Hofrichter, M.; et al. Effects of Forest Management Practices in Temperate Beech Forests on Bacterial and Fungal Communities Involved in Leaf Litter Degradation. Microb. Ecol. 2015, 69, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Ren, H.Y.; Li, M.H.; VanRuijven, J.; Han, X.G.; Wan, S.Q.; Li, H.; Yu, Q.; Jiang, Y.; Jiang, L.; et al. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J. Ecol. 2015, 103, 1308–1316. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stephen, J.R. Ammonia-Oxidizing Bacteria: A model for molecular microbial ecology. Annu. Rev. Microbiol. 2001, 55, 485–529. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Guerrero, M.; Ormeño-Orrillo, E.; Rosenblueth, M.; Martinez-Romero, J.; Martinez-Romero, E. Buffet hypothesis for microbial nutrition at the rhizosphere. Front. Plant Sci. 2013, 4, 188. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.J.; Zhao, F.Z.; Kang, D.; Yang, G.H.; Han, X.H.; Tong, X.G.; Feng, Y.Z.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Lin, C.F.; Peng, J.Q.; Hong, H.B.; Yang, Z.J.; Yang, Y.S. Progress of research on the effect of N, P nutrient effectiveness on the decomposition of forest litter. Acta Ecol. Sin. 2017, 37, 54–62. [Google Scholar]
- Zhang, X.Y.; Ren, H.J.; Zhang, J. Composition and Functional Diversity of Microbial Community across a Mangrove-Inhabited Mudflat as revealed by 16S rRNA gene sequences. Sci. Total Environ. 2018, 633, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Huang, Y.M.; An, S.S.; Zeng, Q.C.; Wang, B.R.; Bai, X.J.; Huang, Q. Decay stages and meteorological factors affect microbial community during leaf litter in situ decomposition. Soil. Ecol. Lett. 2023, 5, 220160. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Wang, J.; Zhang, Y.; Xiao, C. Effects of warming on the bacterial community and its function in a temperate steppe. Sci. Total Environ. 2021, 792, 148409–148416. [Google Scholar] [CrossRef]
- Xiang, X.; Man, B.Y.; Zhan, J.Z.; Luo, Y.; Mao, X.Y.; Zhang, C.; Sun, B.H.; Wang, X. Vertical distribution of bacterial community and functional groups mediating nitrogen cycling in mount Huangshan, Anhui, China. Ecol. Environ. 2023, 32, 56–69. [Google Scholar]
- Li, Y.B.; Sun, X.X.; Yang, R.; Guo, L.F.; Li, C.B.; Wang, X.Y.; Li, B.Q.; Liu, H.Q.; Wang, Q.; Soleimani, M.; et al. Phototrophic nitrogen fixation, a neglected biogeochemical process in Mine Tailings? Environ. Sci. Technol. 2024, 58, 6192–6203. [Google Scholar] [CrossRef] [PubMed]



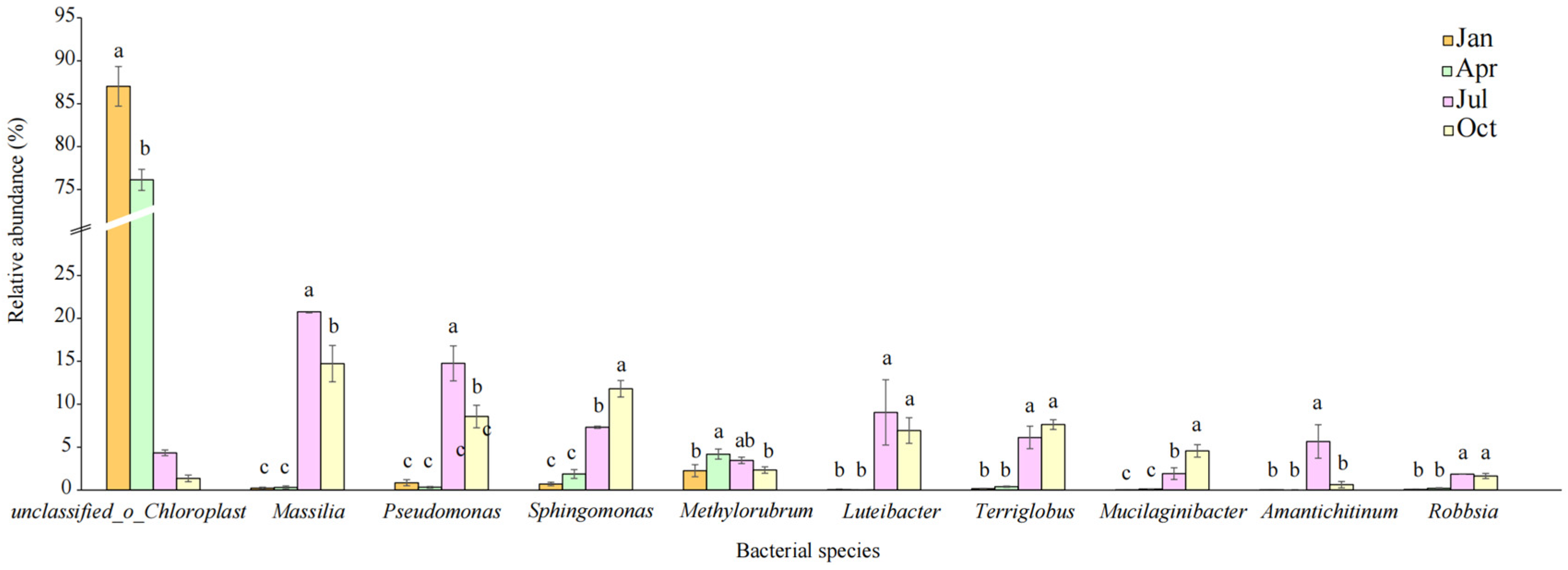


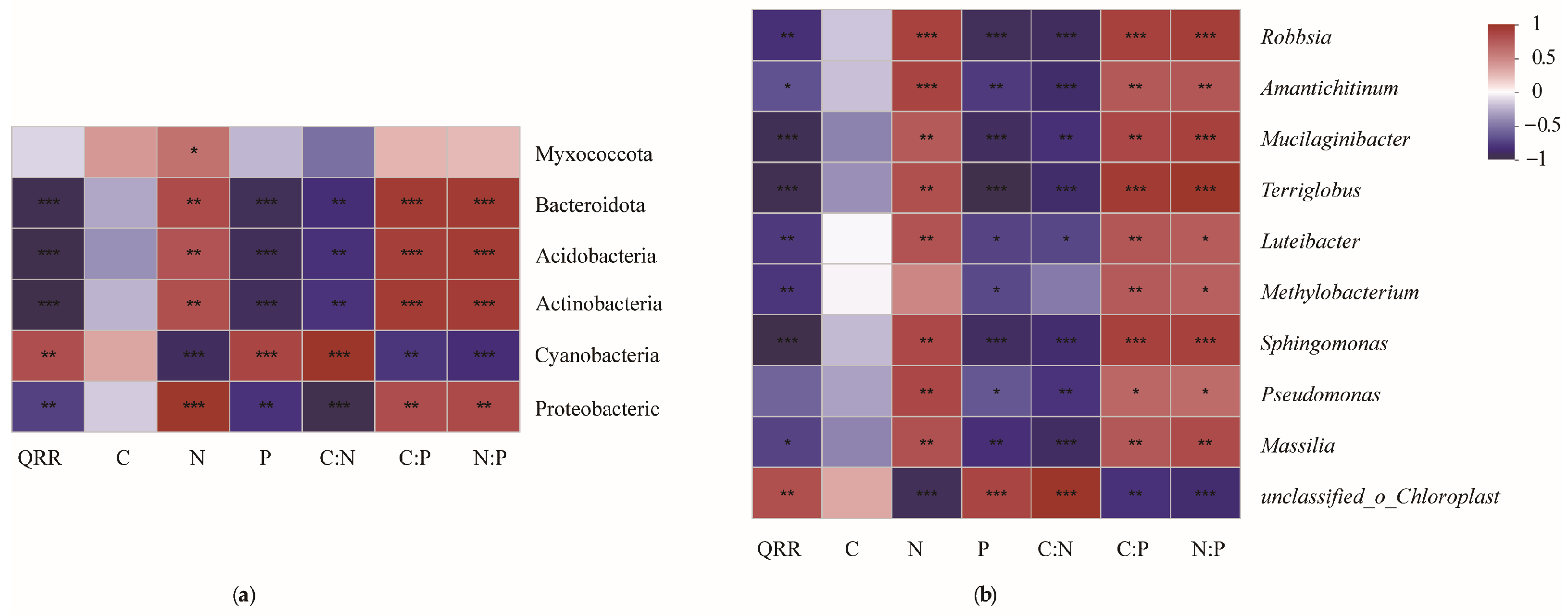
| Sampling Date | Quality Residual Rate (%) | C (g kg−1) | N (g kg−1) | P (g kg−1) | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|
| Initial content (0 d) | 100 a | 48.91 ± 0.23 a | 1.67 ± 0.04 c | 3.01 ± 0.03 a | 29.25 ± 0.8 a | 16.27 ± 0.24 b | 0.56 ± 0.02 c |
| January (92 d) | 89.60 ± 0.11 b | 49.63 ± 0.39 a | 2.09 ± 0.05 b | 3.04 ± 0.05 a | 23.79 ± 0.36 b | 16.34 ± 0.35 b | 0.69 ± 0.02 c |
| April (182 d) | 87.30 ± 0.35 c | 49.79 ± 0.28 a | 2.37 ± 0.01 a | 2.84 ± 0.02 ab | 21.02 ± 0.16 c | 17.53 ± 0.04 b | 0.83 ± 0.01 b |
| July (273 d) | 82.03 ± 0.51 d | 49.18 ± 0.45 a | 2.45 ± 0.00 a | 2.63 ± 0.04 bc | 20.06 ± 0.2 c | 18.73 ± 0.14 ab | 0.93 ± 0.01 ab |
| October (365 d) | 77.57 ± 0.51 e | 49.27 ± 0.08 a | 2.42 ± 0.01 a | 2.37 ± 0.21 c | 20.33 ± 0.11 c | 21.14 ± 1.85 a | 1.04 ± 0.09 a |
| Sampling Date | Sobs | Shannon Index | ACE Index | Pd |
|---|---|---|---|---|
| January (92 d) | 195 ± 35.34 c | 0.79 ± 0.128 d | 368.3 ± 50.39 c | 19.77 ± 3.63 c |
| April (182 d) | 336 ± 25.12 b | 1.409 ± 0.048 c | 536.5 ± 83.1 b | 34.71 ± 1.93 b |
| July (273 d) | 350.7 ± 52.35 b | 3.28 ± 0.212 b | 674.2 ± 66.6 ab | 31.17 ± 3.15 b |
| October (365 d) | 468.7 ± 22.81 a | 3.888 ± 0.053 a | 705.6 ± 103.8 a | 42 ± 5.73 a |
| Functional Groups | January | April | July | October |
|---|---|---|---|---|
| chloroplasts | 90.69 ± 1.04 a | 83.04 ± 1.09 b | 0.64 ± 0.06 c | 1.02 ± 0.29 c |
| achemoheterotrophy | 2.39 ± 0.28 c | 4.92 ± 0.3 b | 23.93 ± 2.66 a | 24.73 ± 2.55 a |
| aerobic chemoheterotrophy | 2.07 ± 0.24 c | 3.66 ± 0.58 b | 23.00 ± 1.75 a | 24.09 ± 2.49 a |
| ureolysis | 0.23 ± 0.04 c | 0.87 ± 0.10 b | 13.61 ± 0.47 a | 11.69 ± 1.39 a |
| animal parasites or symbionts | 0.06 ± 0.01 c | 0.18 ± 0.05 b | 11.95 ± 1.59 a | 10.81 ± 1.19 a |
| human pathogens all | 0.06 ± 0.01 c | 0.15 ± 0.02 b | 11.93 ± 1.59 a | 10.81 ± 1.19 a |
| human pathogens pneumonia | 0.02 ± 0.00 b | 0.03 ± 0.00 b | 12.59 ± 0.72 a | 10.77 ± 1.19 a |
| intracellular parasites | 3.45 ± 0.40 a | 3.47 ± 0.47 a | 0.38 ± 0.03 b | 0.57 ± 0.06 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Cheng, J.; Cao, H.; Yang, F.; Jiang, M.; Li, M.; Huang, Q. Fast Bacterial Succession Associated with the Decomposition of Larix gmelinii Litter in Wudalianchi Volcano. Microorganisms 2024, 12, 948. https://doi.org/10.3390/microorganisms12050948
Xie L, Cheng J, Cao H, Yang F, Jiang M, Li M, Huang Q. Fast Bacterial Succession Associated with the Decomposition of Larix gmelinii Litter in Wudalianchi Volcano. Microorganisms. 2024; 12(5):948. https://doi.org/10.3390/microorganisms12050948
Chicago/Turabian StyleXie, Lihong, Jiahui Cheng, Hongjie Cao, Fan Yang, Mingyue Jiang, Maihe Li, and Qingyang Huang. 2024. "Fast Bacterial Succession Associated with the Decomposition of Larix gmelinii Litter in Wudalianchi Volcano" Microorganisms 12, no. 5: 948. https://doi.org/10.3390/microorganisms12050948





