Green Manuring Enhances Soil Multifunctionality in Tobacco Field in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analysis of Soil Physical and Chemical Properties
2.3. Soil DNA Extraction and PCR Amplification
2.4. Illumina MiSeq Sequencing
2.5. Bioinformatic Analysis
2.6. Data Analysis
2.6.1. Soil Multifunctionality
2.6.2. Microbial Co-Occurrence Network Analysis
2.6.3. Statistical Analysis
3. Results
3.1. Effects of Different Green Manures on Tobacco Biomass, Soil Properties, and Extracellular Enzyme Activity
3.2. The Composition and Structure of Rhizosphere Microbial Communities in Response to Different Green Manures
3.3. The Effect of Different Green Manures on Soil Multifunctionality
3.4. Soil Bacterial–Fungal Symbiotic Network Analysis under Different Green Manure Treatments
4. Discussion
4.1. Effect of Green Manure Application on Soil Nutrients and Extracellular Enzyme Activities
4.2. Response of Microbial Diversity to Different Green Manures
4.3. The Effect of Different Green Manures on Network Modules
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Faucon, M.-P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M.K.; Dong, L.; Akram, M.A.; Etldesoky, G.E.; Aljuwayid, A.M.; Chuah, L.F.; Deng, J. Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Fan, K.; Gao, S.; Chang, D.; Li, G.; Liang, T.; Liang, H.; Li, S.; Zhang, J.; Che, Z.; et al. Green manuring relocates microbiomes in driving the soil functionality of nitrogen cycling to obtain preferable grain yields in thirty years. Sci. China-Life Sci. 2023, 67, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, S.; Zhou, G.; Fu, L.; Hu, F.; Gao, S.; Cao, W. Targeted Regulation of the Microbiome by Green Manuring to Promote Tobacco Growth. Biol. Fertil. Soils 2024, 60, 69–85. [Google Scholar] [CrossRef]
- Huang, Q.; Gong, Y.; Dewi, R.K.; Li, P.; Wang, X.; Hashimi, R.; Komatsuzaki, M. Enhancing energy efficiency and reducing carbon footprint in organic soybean production through no-tillage and rye cover crop integration. J. Clean. Prod. 2023, 419, 138247. [Google Scholar] [CrossRef]
- Romero, F.; Hilfiker, S.; Edlinger, A.; Held, A.; Hartman, K.; Labouyrie, M.; van der Heijden, M.G. Soil microbial biodiversity promotes crop productivity and agro-ecosystem functioning in experimental microcosms. Sci. Total Environ. 2023, 885, 163683. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Duan, Y.; Lei, X.; Cao, Y.; Liu, L.; Shang, X.; Wang, M.; Lv, C.; Ma, Y.; Fang, W.; et al. Tea Plantation Intercropping Legume Improves Soil Ecosystem Multifunctionality and Tea Quality by Regulating Rare Bacterial Taxa. Agronomy 2023, 13, 1110. [Google Scholar] [CrossRef]
- Luo, J.; Liao, G.; Banerjee, S.; Gu, S.; Liang, J.; Guo, X.; Zhao, H.; Liang, Y.; Li, T. Long-term organic fertilization promotes the resilience of soil multifunctionality driven by bacterial communities. Soil Biol. Biochem. 2023, 177, 108922. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.G.; Chu, H. Microbial resistance promotes plant production in a four-decade nutrient fertilization experiment. Soil Biol. Biochem. 2020, 141, 107679. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Wang, F.; Han, W.; Qiao, M.; Dong, W.; Hu, C.; Zhu, D.; Chu, H.; Zhu, Y.G. The ecological clusters of soil organisms drive the ecosystem multifunctionality under long-term fertilization. Environ. Int. 2022, 161, 107133. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhou, J.; Chu, J.; Shahbaz, M.; Yang, Y.; Jones, D.L.; Zang, H.; Razavi, B.S.; Zeng, Z. Insights into the associations between soil quality and ecosystem multifunctionality driven by fertilization management: A case study from the North China Plain. J. Clean. Prod. 2022, 362, 132265. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; de Beeck, M.O.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Gao, S. Chinese Green Manure Development Strategy by 2025. Chin. J. Agric. Resour. Reginal Plan. 2023, 44, 1–9. [Google Scholar]
- Gao, S.; Cao, W.; Zhou, G.; Rees, R.M. Bacterial communities in paddy soils changed by milk vetch as green manure: A study conducted across six provinces in South China. Pedosphere 2021, 31, 521–530. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co-incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Tillage Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, D.; Hu, A.; Wang, Y.; Sun, Y.; Li, C. Effects of green manure intercropping on soil nutrient content and bacterial community structure in litchi orchards in China. Front. Environ. Sci. 2023, 10, 1059800. [Google Scholar] [CrossRef]
- Couëdel, A.; Alletto, L.; Justes, E. The acquisition of macro- and micronutrients is synergistic in species mixtures: Example of mixed crucifer-legume cover crops. Front. Agron. 2023, 5, 1223639. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xie, J.P.; Li, Z.H. Regionalization of Tobacco Planting in China; Science Press: Beijing, China, 2000; pp. 36–37. [Google Scholar]
- Yang, J.H.; Yu, J.L. Advance in obstruction factor and solution ways of tobacco continuous cropping. Hunan Agric. Sci. 2016, 8, 113–116. [Google Scholar]
- Li, Z.J.; Zhu, W.Q.; Huang, K.; Ji, X.W.; Wang, C.; Zhao, T.; Chen, J.H.; Ye, X.K.; Ma, L.; Pan, Y.H. Influences of continuous cropping on agronomic characters, root morphology and soil nutrients of flue-cured tobacco. Jiangsu Agric. Sci. 2022, 50, 67–72. [Google Scholar]
- Fu, Z.Y.; Zhang, X.Y.; Zhang, X.F.; Zhou, H.J.; Qin, Y.H.; Ma, J.; Han, Q.J.; Ye, X.F. Effect of continuous cropping on quality of flue-cured tobacco leaves and carbon pool in tobacco growing soil. J. Northwest AF Univ. (Nat. Sci. Ed.) 2018, 46, 16–22. [Google Scholar]
- Liu, Q.Z.; Guo, F.Y.; Wu, Z.H.; Zhu, J.W. Controlling measures and factors of fuel-tobacco continuous cropping. Chin. Agric. Sci. Bull. 2012, 28, 87–90. [Google Scholar]
- Cao, W.; Bao, X.; Xu, C.; Nie, J.; Gao, Y.; Geng, M. Reviews and prospects on science and technology of green manure in China. J. Plant Nutr. Fertil. 2017, 23, 1450–1461. [Google Scholar]
- Si, G.; Wu, W.; Mei, D.; Tan, J.; Tian, Y.; Yuan, J.; Zhao, S. Effect of different burying amount of vicia villosa on yield and quality of flue-cured tobacco. Chin. Tob. Sci. 2011, 32, 82–86. [Google Scholar]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.G.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G. The Synergistic Effects and Mechanism of Co-Incorporating Chinese Milk Vetch (Astragalus sinicus L.) and Rice (Oryza sativa L.) Straw. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Gundale, M.J.; Farrell, M.; Ciobanu, M.; Baldock, J.A.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Consistent effects of biodiversity loss on multifunctionality across contrasting ecosystems. Nat. Ecol. Evol. 2018, 2, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.G.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; Blanchet, F.G.; Minchin, P.R.; O’Hara, R.B. Package ‘Vegan’: Community Ecology Package, R Package Version 2.2-1; R Foundation: Vienna, Austria, 2007. [Google Scholar]
- Fauzan, M.I. The Effect of NPK compound fertilizer and green manure on soil pH, exchangeable K, cation exchange capacity on ultisols jatinangor. Int. J. Sci. Res. Publ. 2023, 13, 141–145. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Jia, X.; Xu, C. Influence of physico-chemical property of soil and the tobacco aroma composition by using organic fertilizer in the tobacco farmland. Acta Tabacaria Sin. 2005, 11, 29–33. [Google Scholar]
- Legendre, P.; Borcard, D.; Peres-Neto, P.R. Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol. Monogr. 2005, 75, 435–450. [Google Scholar] [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 2016, 99, 137–149. [Google Scholar] [CrossRef]
- Zhou, J.; Qiao, N.; Zhu, T.; Pang, R.; Sun, Y.; Zhou, X.; Xu, X. Native soil labile organic matter influences soil priming effects. Appl. Soil Ecol. 2023, 182, 104732. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Trivedi, P.; Trivedi, C.; Eldridge, D.J.; Reich, P.B.; Jeffries, T.C.; Singh, B.K. Microbial richness and composition independently drive soil multifunctionality. Funct. Ecol. 2017, 31, 2330–2343. [Google Scholar] [CrossRef]
- Anantharaman, K.; Hausmann, B.; Jungbluth, S.P.; Kantor, R.S.; Lavy, A.; A Warren, L.; Rappé, M.S.; Pester, M.; Loy, A.; Thomas, B.C.; et al. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J. 2018, 12, 1715–1728. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Chu, H.; Eldridge, D.J.; Gaitan, J.J.; Liu, Y.-R.; Sokoya, B.; Wang, J.-T.; Hu, H.-W.; He, J.-Z.; Sun, W.; et al. Soil biodiversity supports the delivery of multiple ecosystem functions in urban greenspaces. Nat. Ecol. Evol. 2023, 7, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, S.; Ma, L.; Zhou, J.; Huang, Y.; Zhang, J.; Wang, L.; Guo, L.; Tian, C. Community assembly processes of soil bacteria and fungi along a chronosequence of rice paddies cultivated on saline-sodic land. Land Degrad. Dev. 2023, 34, 3648–3662. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, L.; Chen, B.; Fu, W.; Xie, W.; Xu, T.; Zhang, W.; Ye, Q.; Feng, H.; Fu, S. Soil fungal community is more sensitive to nitrogen deposition than increased rainfall in a mixed deciduous forest of China. Soil Ecol. Lett. 2020, 1, 20–32. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.S.; Chandrasekhara Rao, C.; Raju, C.A. Genetic variability in Fusarium oxysporum isolates causing wilt of tobacco using RAPD markers. J. Mycol. Plant Pathol. 2009, 39, 141–143. [Google Scholar]
- Xu, J.; Xu, X.-D.; Cao, Y.-Y.; Zhang, W.-M. First report of greenhouse tomato wilt caused by Plectosphaerella cucumerina in China. Plant Dis. 2014, 98, 158. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.C.; Fegan, N.; Fegan, M.; Stirling, G.R. Stenotrophomonas and Lysobacter: Ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 2010, 108, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, F.; Yao, H. Response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. J. Soils Sediments 2019, 19, 1948–1958. [Google Scholar] [CrossRef]
- Pandit, S.G.; Ramesh, K.P.M.; Puttananjaiah, M.H.; Dhale, M.A. Cicer arietinum (Bengal grem) husk as alternative for Talaromyces purpureogenus CFRM02 pigment production bioactivities and identification. LWT 2019, 116, 108499. [Google Scholar] [CrossRef]
- Luo, Y. Effects of Human Activities on Microbial Communities and Antibiotic Resistance Genes in Sediment of Jialing River. Master’s Thesis, China West Normal University, Nanchong, China, 2021. [Google Scholar]
- Sun, J. Effects of Exogenous Additives on Hormesis Effect of Cd Induced Soil Respiration. Master’s Thesis, Nanjing Forest University, Nanjing, China, 2022. [Google Scholar]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Himmelstein, J.; Maul, J.E.; Balci, Y.; Everts, K.L. Factors Associated with Leguminous Green Manure Incorporation and Fusarium Wilt Suppression in Watermelon. Plant Dis. 2016, 100, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, S.-J.; Cao, W.; Duan, T. Research progress on the influence of green manures on soil-borne diseases in farmlands. Acta Agrestia Sin. 2021, 29, 1605–1614. [Google Scholar]


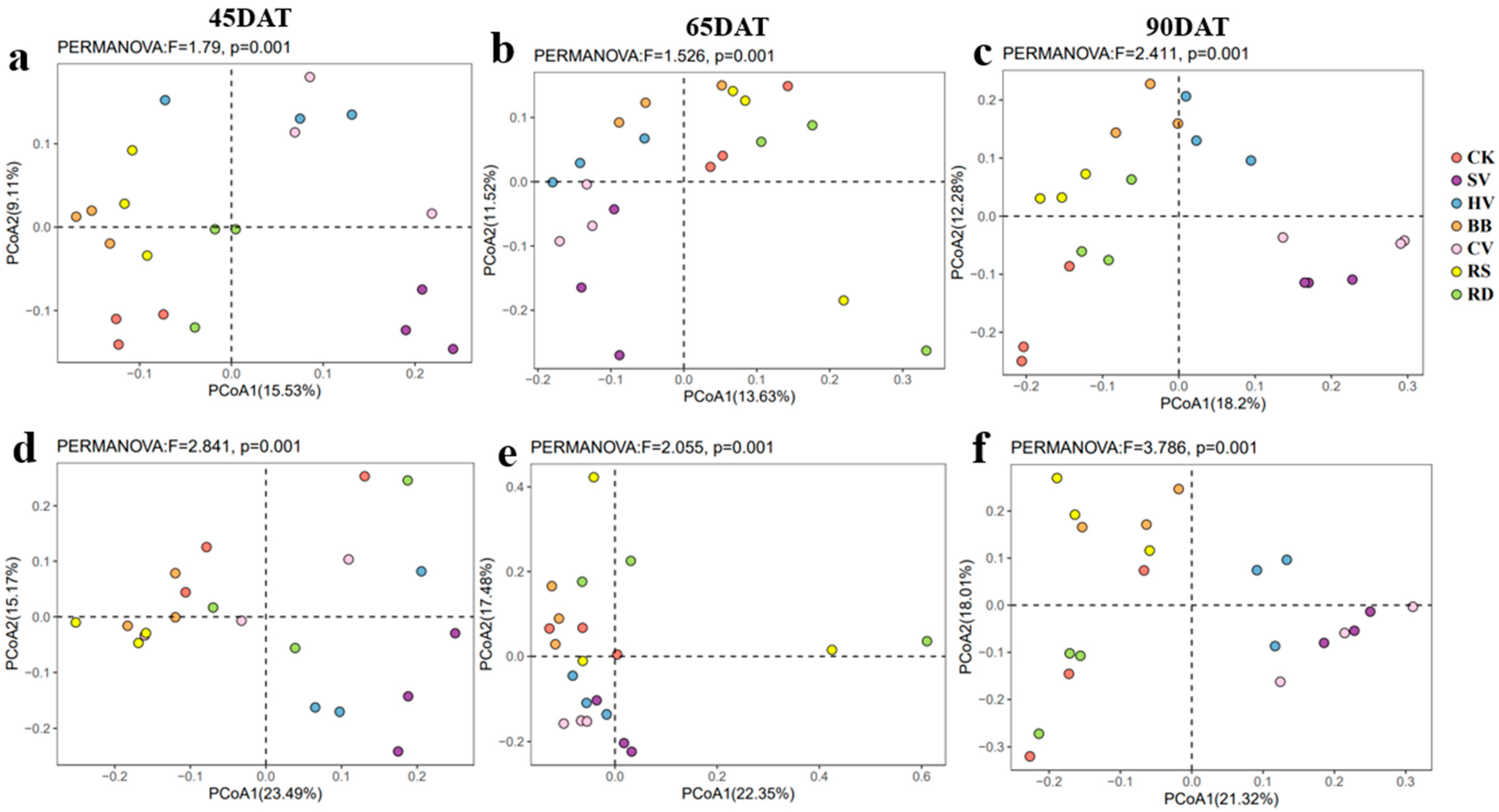



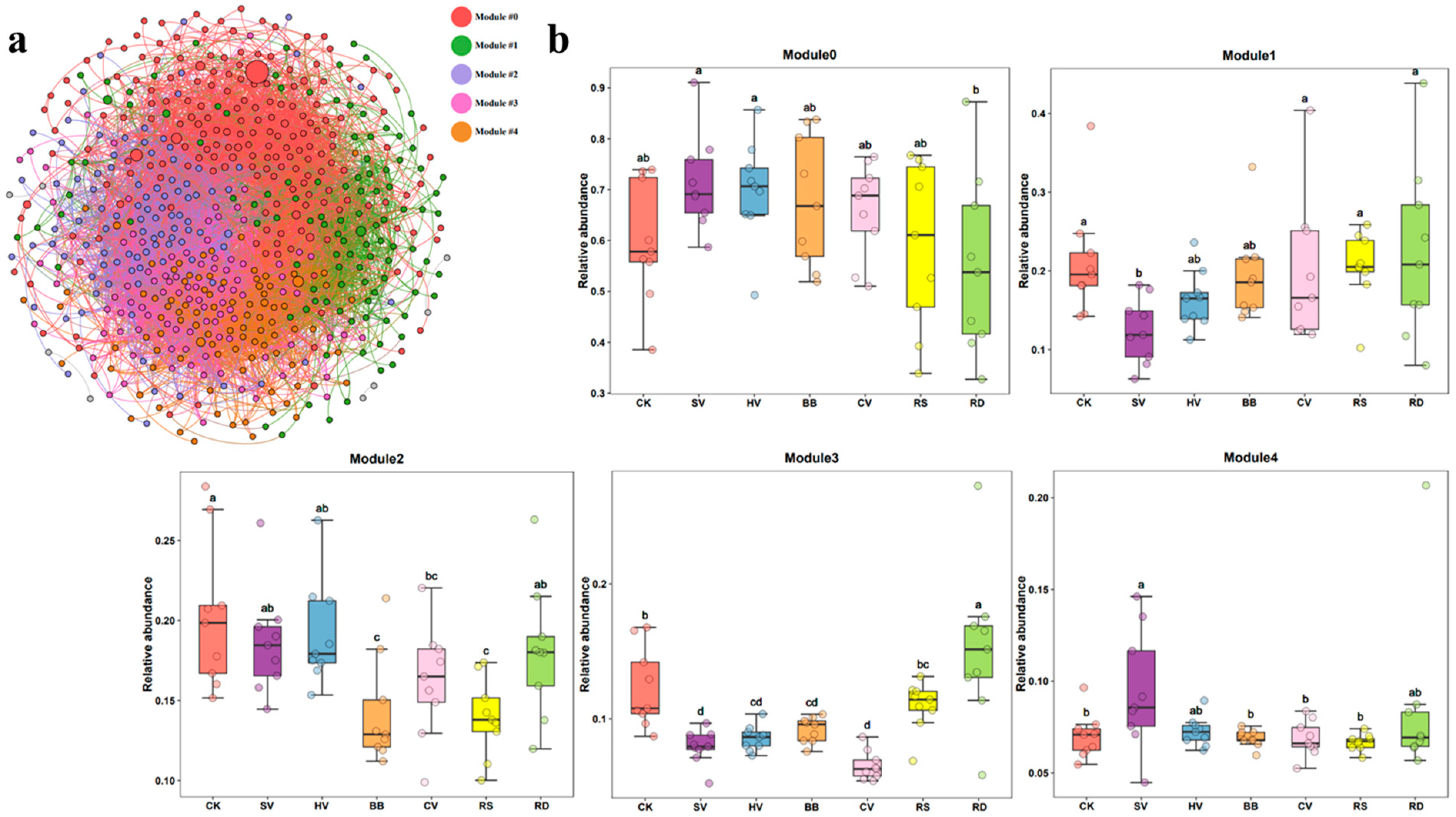





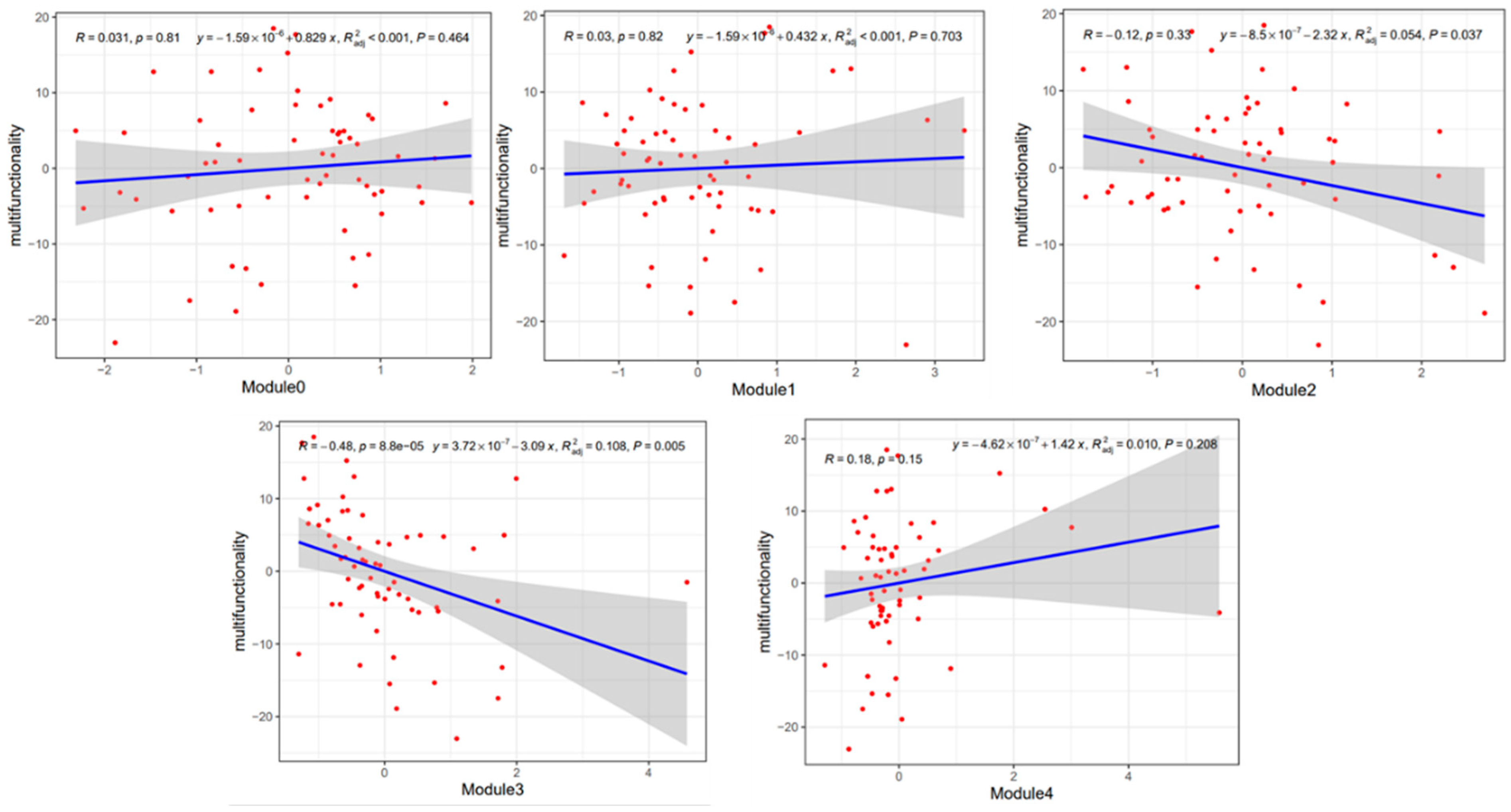
| Stage | Treatment | pH | SOM (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | DOC (mg kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 45DAT | CK | 6.82 a | 36.47 d | 2.20 e | 1.73 c | 9.19 b | 44 ab | 1.62 ab | 28.5 ab | 88 e | 750 b |
| SV | 6.6 bc | 38.53 c | 2.50 d | 2.02 b | 9.73 ab | 32.1 ab | 1.72 ab | 51.5 ab | 118.5 cd | 1516.7 a | |
| HV | 6.41 d | 41.34 b | 2.79 b | 2.31 a | 9.7 ab | 41.1 ab | 2.21 ab | 38.2 ab | 156 ab | 1436.7 a | |
| BB | 6.49 cd | 45.59 a | 2.97 a | 2.26 a | 10 ab | 41 b | 1.11 b | 27 b | 174 a | 1550 a | |
| CV | 6.69 ab | 41.5 b | 2.72 bc | 1.97 b | 9.29 ab | 36.5 a | 4.8 a | 39.7 a | 143.17 bc | 1530 a | |
| RS | 6.69 ab | 44.27 a | 2.74 b | 2.02 b | 9.63 ab | 42.7 b | 0.26 b | 20 b | 123.83 cd | 1303.3 a | |
| RD | 6.65 bc | 41.82 b | 2.59 cd | 2.17 ab | 10.08 a | 35.5 ab | 1.84 ab | 42.2 ab | 107.83 de | 1790 a | |
| 65DAT | CK | 6.90 ab | 36.1 d | 2.24 e | 1.79 c | 8.82 a | 34.6 a | 0.05 b | 11.1 b | 79.2 e | 576.7 e |
| SV | 6.63 cd | 38.3 c | 2.48 d | 1.91 c | 9.18 a | 49.5 a | 0.33 ab | 38.3 ab | 84.8 de | 903.3 de | |
| HV | 6.52 d | 40.9 b | 2.64 c | 2.24 ab | 9.37 a | 36.7 a | 2.59 a | 51.3 a | 125.3 bcd | 1013.3 cde | |
| BB | 6.74 bc | 45.4 a | 3.07 a | 2.45 a | 9.93 a | 46.1 a | 0.3 ab | 23.5 ab | 168.2 a | 1556.7 ab | |
| CV | 6.67 cd | 43.2 a | 3.02 a | 2.23 ab | 9.85 a | 50.1 a | 1.08 ab | 42.2 ab | 154.8 ab | 2050 a | |
| RS | 6.77 bc | 45 a | 2.83 b | 2.06 bc | 9.99 a | 37 a | 0.97 ab | 29.3 ab | 127.3 abc | 1520 bc | |
| RD | 6.94 a | 40.7 b | 2.66 c | 2.03 bc | 8.86 a | 43.9 a | 1.35 ab | 46.3 ab | 88.2 cde | 1420 bcd | |
| 90DAT | CK | 6.94 a | 34 e | 2.06 c | 1.55 d | 7.77 d | 29.3 b | 0.03 c | 11.1 c | 47.2 d | 188.7 c |
| SV | 6.5 b | 38.2 d | 2.52 b | 1.95 bc | 8.85 ab | 33.7 b | 0.77 ab | 124.7 a | 76.5 c | 910 b | |
| HV | 6.58 b | 41 bc | 2.47 b | 2.28 a | 8.42 bc | 36.9 b | 0.35 bc | 32.2 bc | 118.8 b | 836.7 b | |
| BB | 6.94 a | 45.8 a | 2.8 a | 2.27 a | 8.22 cd | 35 b | 0.18 c | 28 bc | 139.8 a | 413.3 c | |
| CV | 6.53 b | 42 b | 2.84 a | 2.07 abc | 8.67 bc | 50.6 a | 0.87 a | 46.7 bc | 118.2 b | 1066.7 b | |
| RS | 7.12 a | 44.1 a | 2.54 b | 1.8 cd | 7.89 d | 35.3 b | 0.13 c | 32.6 bc | 74.7 c | 269.3 c | |
| RD | 6.56 b | 39.7 cd | 2.59 b | 2.16 ab | 9.24 a | 30.1 b | 0.41 abc | 58.8 b | 115.5 b | 1406.7 a |
| Stage | Treatment | AG | BG | BC | BX | NAG | LA | AP |
|---|---|---|---|---|---|---|---|---|
| 45DAT | CK | 3.72 a | 26.9 ab | 3.27 ab | 3.00 b | 6.65 b | −84.01 c | 22.3 ab |
| SV | 6.14 a | 45.9 a | 5.53 ab | 6.29 a | 17.4 a | 3.83 b | 59.9 a | |
| HV | 3.61 a | 26.3 ab | 3.11 b | 3.41 ab | 11.1 ab | 12.78 b | 57 ab | |
| BB | 4.11 a | 19.8 b | 2.14 b | 2.53 b | 9.10 b | −78.88 c | 19.9 b | |
| CV | 6.25 a | 51.6 a | 7.88 a | 5.99 a | 16.7 a | 97.73 a | 43 ab | |
| RS | 4.7 a | 29.3 ab | 3.72 ab | 4.45 ab | 13.4 ab | 5.35 b | 28.7 ab | |
| RD | 3.65 a | 26.9 ab | 3.03 b | 3.44 ab | 11 ab | 9.93 b | 35.6 ab | |
| 65DAT | CK | 4.11 a | 27.2 b | 3.9 b | 3.73 ab | 9.2 b | 35 b | 28.9 c |
| SV | 2.31 a | 32.8 b | 3.33 b | 3.11 b | 18.9 ab | 34.9 b | 34.2 bc | |
| HV | 4.87 a | 49.9 ab | 6.23 ab | 6.18 ab | 17 ab | 202.7 a | 106.8 a | |
| BB | 6.8 a | 38 b | 6.3 ab | 7.22 ab | 14.5 ab | −9.2 b | 73.6 abc | |
| CV | 6.36 a | 69.6 a | 9.85 a | 9.23 a | 26.5 a | 27.6 b | 95.3 ab | |
| RS | 5.92 a | 44.3 ab | 5.86 ab | 6.09 ab | 15.3 ab | 265.8 a | 34.7 bc | |
| RD | 5.54 a | 50.5 ab | 7.97 ab | 6.03 ab | 24.6 a | 200.3 a | 43.2 abc | |
| 90DAT | CK | 7.2 c | 33.2 c | 3.4 c | 4.91 c | 8.43 c | 166.3 d | 41.4 c |
| SV | 30.9 a | 154 a | 23.9 a | 17.39 ab | 40.8 ab | 328.4 a | 187.6 a | |
| HV | 10.7 bc | 51.6 c | 7.1 bc | 8.14 c | 19.1 c | 235 bc | 81.6 c | |
| BB | 12 bc | 54.3 c | 6.8 bc | 8.24 c | 19.4 c | 206.4 d | 109.1 bc | |
| CV | 22.9 ab | 109.4 ab | 15.5 ab | 21.49 a | 42.2 a | 239 bc | 167 ab | |
| RS | 13.3 bc | 60.9 bc | 7.7 bc | 8.15 c | 19.3 c | 224.5 bcd | 54.2 c | |
| RD | 16.9 abc | 72.2 bc | 10.5 bc | 11.16 bc | 24 bc | 240.4 b | 105.6 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Chen, H.; Fu, L.; Yin, M.; Wang, Z.; Li, Y.; Cao, W. Green Manuring Enhances Soil Multifunctionality in Tobacco Field in Southwest China. Microorganisms 2024, 12, 949. https://doi.org/10.3390/microorganisms12050949
Feng Y, Chen H, Fu L, Yin M, Wang Z, Li Y, Cao W. Green Manuring Enhances Soil Multifunctionality in Tobacco Field in Southwest China. Microorganisms. 2024; 12(5):949. https://doi.org/10.3390/microorganisms12050949
Chicago/Turabian StyleFeng, Yu, Hua Chen, Libo Fu, Mei Yin, Zhiyuan Wang, Yongmei Li, and Weidong Cao. 2024. "Green Manuring Enhances Soil Multifunctionality in Tobacco Field in Southwest China" Microorganisms 12, no. 5: 949. https://doi.org/10.3390/microorganisms12050949





