Enhancing Growth and Gut Health in Squabs: The Impact of Fermented Mixed Feed
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of FMF
2.2. Experimental Design
2.3. Real-Time Quantitative PCR (RT-qPCR)
2.4. Histopathological Analysis
2.5. Gut Microbiome and Metabolomic Analyses
2.6. Statistical Analyses
3. Results
3.1. Growth and Carcass Performance of Squabs
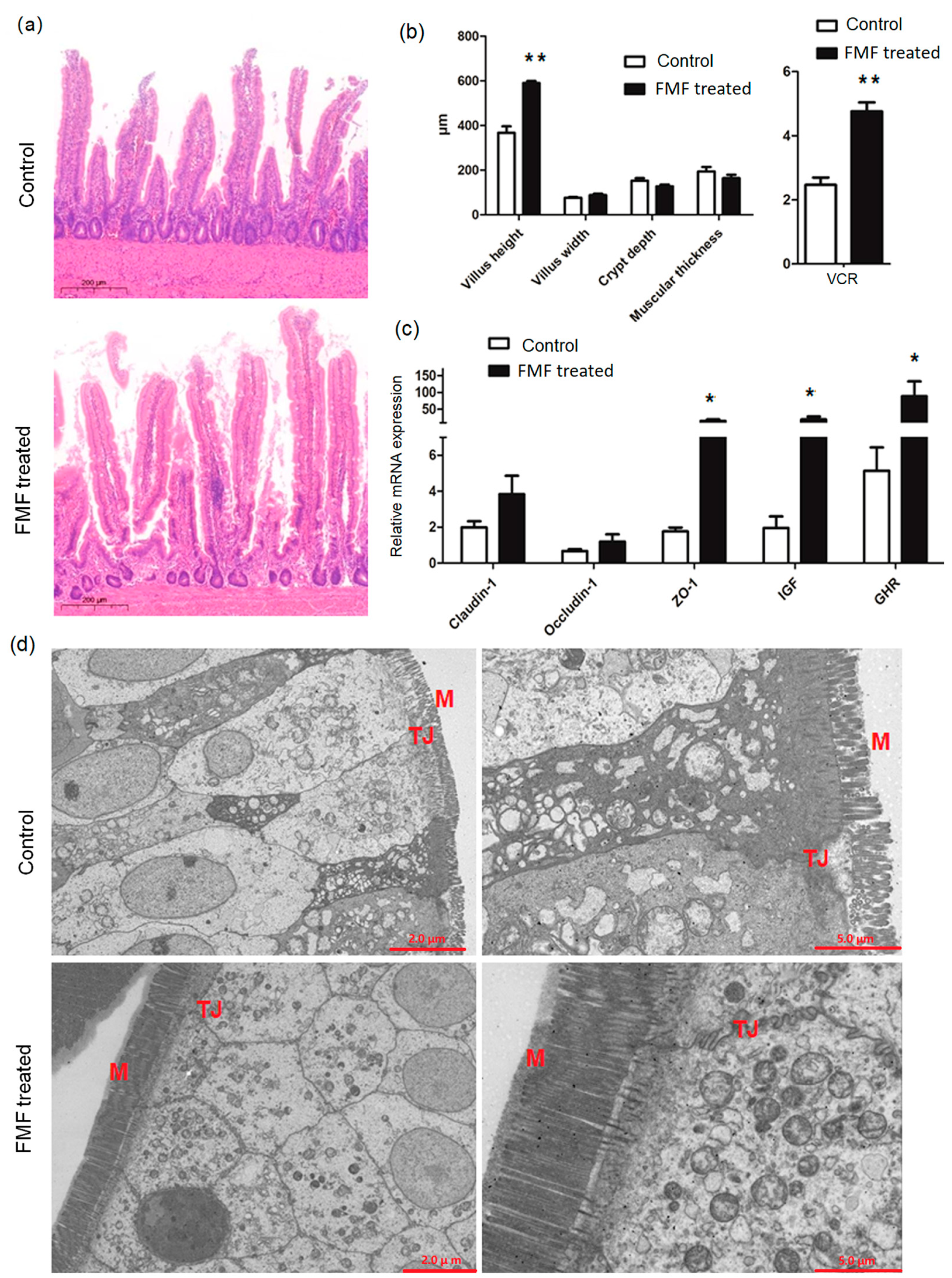
3.2. H&E Staining, qRT-PCR, and TEM
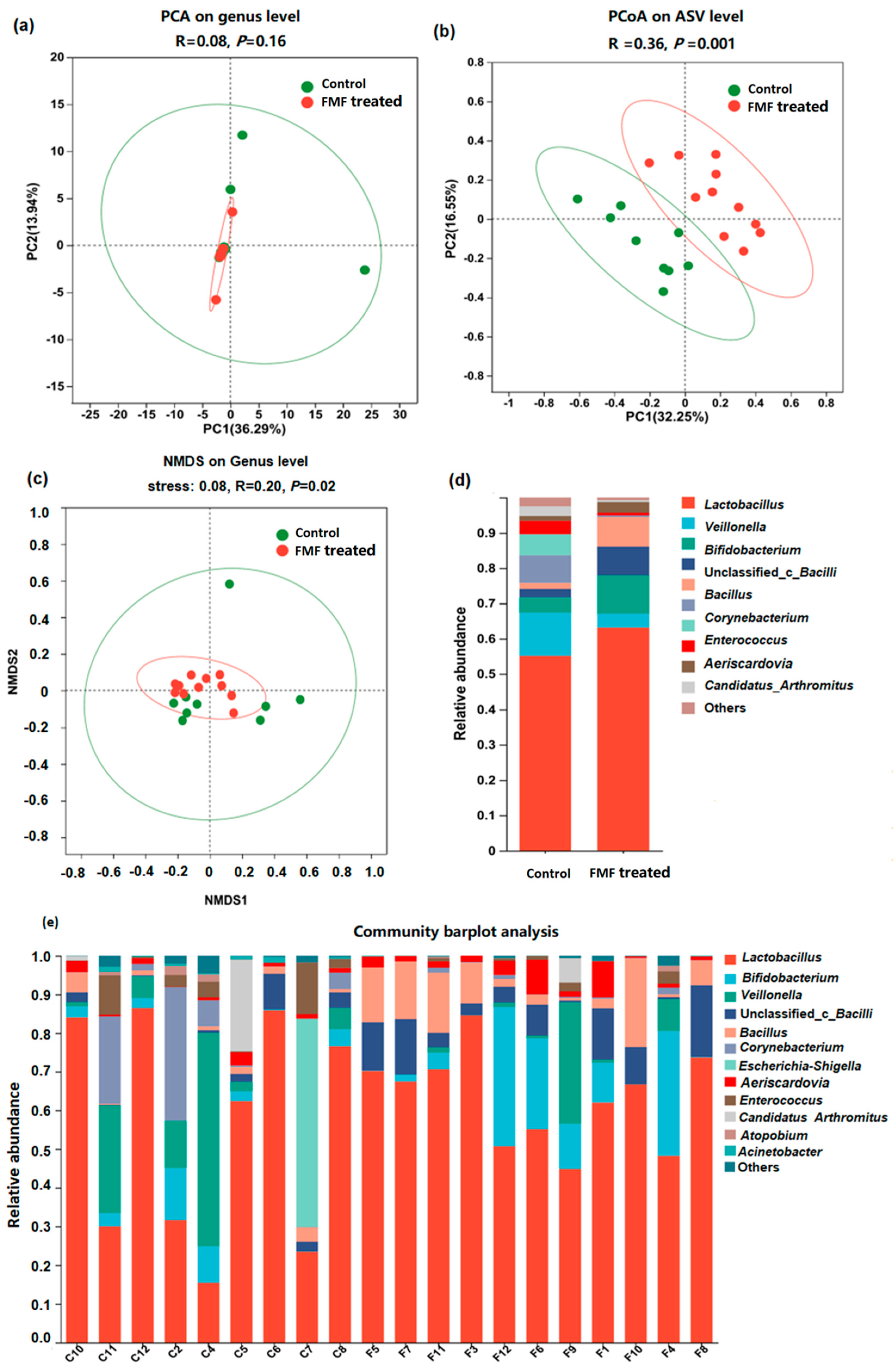
3.3. Taxonomic Analysis
3.4. Metabolite Analysis
3.5. Analysis of Correlations between Metabolites and Microorganisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, M.; Xu, M.; Chen, C.; He, Y.; Ding, M.; Ding, X.; Wei, W.; Yang, S.; Zhou, B. Expression analyses of candidate genes related to meat quality traits in squabs from two breeds of meat-type pigeon. J. Anim. Physiol. Anim. Nutr. 2018, 102, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Pomianowski, J.F.; Mikulski, D.; Pudyszak, K.; Cooper, R.G.; Angowski, M.; Jozwik, A.; Horbanczuk, J.O. Chemical composition, cholesterol content, and fatty acid profile of pigeon meat as influenced by meat-type breeds. Poult. Sci. 2009, 88, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Ma, T.; Zhong, Y.; Deng, S.; Zhu, S.; Fu, Z.; Huang, Y.; Fu, J. Effect of tea polyphenols supplement on growth performance, antioxidation, and gut microbiota in squabs. Front. Microbiol. 2023, 14, 1329036. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.J.; Haring, V.R.; McColl, K.A.; Monaghan, P.; Donald, J.A.; Nicholas, K.R.; Moore, R.J.; Crowley, T.M. Histological and global gene expression analysis of the ‘lactating’ pigeon crop. BMC Genomics 2011, 12, 452. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Ma, Y.; Li, X.K. The biological function of pigeon crop milk and the regulation of its production. Yi Chuan 2017, 39, 1158–1167. [Google Scholar] [PubMed]
- Jin, C.L.; He, Y.A.; Jiang, S.G.; Wang, X.Q.; Yan, H.C.; Tan, H.Z.; Gao, C.Q. Chemical composition of pigeon crop milk and factors affecting its production: A review. Poult. Sci. 2023, 102, 102681. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Farag, M.R.; Sahfi, M.E.; Elnesr, S.S.; Alqaisi, O.; El-Kassas, S.; Al-Wajeeh, A.S.; Taha, A.E.; Abd E-Hack, M.E. Phytochemical characteristics of Paulownia trees wastes and its use as unconventional feedstuff in animal feed. Anim. Biotechnol. 2022, 33, 586–593. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.R.; Kiesz, M. Dietary fermented rapeseed or/and soybean meal additives on performance and intestinal health of piglets. Sci. Rep. 2021, 11, 16952. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, S.S.; Oyeyode, J.O.; Shafik, W.; Sunusi, Z.A.; Adeyemi, A.A. Proximate analysis of poultry-mix formed feed using maize bran as a base. Int. J. Anal. Chem. 2021, 2021, 8894567. [Google Scholar] [CrossRef]
- Qaisrani, S.N.; Van Krimpen, M.M.; Verstegen, M.W.A.; Hendriks, W.H.; Kwakkel, R.P. Effects of three major protein sources on performance, gut morphology and fermentation characteristics in broilers. Br. Poult. Sci. 2020, 61, 43–50. [Google Scholar] [CrossRef]
- Drazbo, A.; Kozlowski, K.; Ognik, K.; Zaworska, A.; Jankowski, J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in turkey. Poult. Sci. 2019, 98, 6161–6169. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, L.N.; Guo, X.J.; Wang, W.; Hao, Q.H.; Wang, S.Y.; Zhu, B.C. The impacts of fermented feed on laying performance, egg quality, immune function, intestinal morphology and microbiota of laying hens in the late laying cycle. Animal 2022, 16, 100676. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, B.; Xi, Y.; Huan, H.; Li, M.; Yu, J.; Zhu, H.; Dai, Z.; Ying, S.; Zhou, W.; et al. Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult. Sci. 2019, 98, 4673–4684. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Talpur, M.Z.; Zeng, Y.; Xie, P.; Li, J.; Wang, S.; Wang, L.; Zhu, X.; Gao, P.; Jiang, Q.; et al. Influence of fermented feed additive on gut morphology, immune status, and microbiota in broilers. BMC Vet. Res. 2022, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Dirzo, R.; Siemens, D.; Brown, P. Ontogenetic switches from plant resistance to tolerance: Minimizing costs with age? Ecol. Lett. 2007, 10, 177–187. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cai, X.; Guo, Q.; Chen, X.; Zhu, S.; Xu, J. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 2013, 110, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Abaci Gunyar, O. Nutritional value of commercial broiler feed supplemented with olive mill waste fermented with probiotic Rhizopus oryzae strains. J. Appl. Microbiol. 2022, 133, 1872–1881. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Ahmed Pirzado, S.; Haile, T.H.; Cai, H.; Liu, G. The effect of fermented and raw rapeseed meal on the growth performance, immune status and intestinal morphology of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2022, 106, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Konkol, D.; Popiela, E.; Opalinski, S.; Lipinska, A.; Tymoszewski, A.; Krasowska, A.; Lukaszewicz, M.; Korczynski, M. Effects of fermented rapeseed meal on performance, intestinal morphology, the viscosity of intestinal content, phosphorus availability, and egg quality of laying hens. Poult. Sci. 2024, 103, 103256. [Google Scholar] [CrossRef]
- Lambo, M.T.; Ma, H.; Zhang, H.; Song, P.; Mao, H.; Cui, G.; Dai, B.; Li, Y.; Zhang, Y. Mechanism of action, benefits, and research gap in fermented soybean meal utilization as a high-quality protein source for livestock and poultry. Anim. Nutr. 2024, 16, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Csernus, B.; Czegledi, L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. Arch. Anim. Breed. 2020, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Early weaning stress induces intestinal microbiota disturbance, mucosal barrier dysfunction and inflammation response activation in pigeon squabs. Front. Microbiol. 2022, 13, 877866. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhao, W.; Li, J.; Hu, C.; Zou, X.; Dong, X. Dietary supplementation of Chitosan Oligosaccharide-Clostridium butyricum synbiotic relieved early-weaned stress by improving intestinal health on pigeon squabs (Columba livia). Front. Immunol. 2022, 13, 926162. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, D.; Shao, Y.; Yu, X.; Liu, X.; Shan, D.; Wang, Z. Changes in the diversity and composition of gut microbiota in pigeon squabs infected with Trichomonas gallinae. Sci. Rep. 2020, 10, 19978. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Ma, X.; Geng, S.; Jiang, X.; Huang, Q.; Hu, C.; Han, X. Intestinal microbiome-metabolome responses to essential oils in piglets. Front. Microbiol. 2018, 9, 1988. [Google Scholar] [CrossRef]
- Guzman, J.R.; Conlin, V.S.; Jobin, C. Diet, microbiome, and the intestinal epithelium: An essential triumvirate? Biomed. Res. Int. 2013, 2013, 425146. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The functional roles of Lactobacillus acidophilus in different physiological and pathological processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021, 71, 2011–2021. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.H.; Yang, G.; Duan, J.L.; Yang, L.Y.; Chen, L.X.; Hou, S.L.; Huang, X.G. Oral administration of Lactobacillus delbrueckii enhances intestinal immunity through inducing dendritic cell activation in suckling piglets. Food Funct. 2022, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Hsu, W.F.; Chang, J.S.; Shih, C.K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Hwang, J.H.; Park, E.O.; Lee, S.O.; Chung, Y.J.; Chung, M.J.; Lim, S.; Lim, T.J.; Ha, Y.; Park, B.H.; et al. Regulation of alcohol and acetaldehyde metabolism by a mixture of Lactobacillus and Bifidobacterium species in human. Nutrients 2021, 13, 1875. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.Y.; Zhang, M.; Tu, W.J.; Zhang, Q.; Jin, M.L.; Fang, R.D.; Jiang, S. Bacillus subtilis inhibits intestinal inflammation and oxidative stress by regulating gut flora and related metabolites in laying hens. Animal 2022, 16, 100474. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kalt, F.; Schulthess, B.; Sidler, F.; Herren, S.; Fucentese, S.F.; Zingg, P.O.; Berli, M.; Zinkernagel, A.S.; Zbinden, R.; Achermann, Y. Corynebacterium species rarely cause orthopedic infections. J. Clin. Microbiol. 2018, 56, e01200-18. [Google Scholar] [CrossRef] [PubMed]
- Hacker, E.; Antunes, C.A.; Mattos-Guaraldi, A.L.; Burkovski, A.; Tauch, A. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016, 11, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Reddy, K.R.; O’Leary, J.G.; Vargas, H.E.; Lai, J.C.; Kamath, P.S.; Tandon, P.; Wong, F.; Subramanian, R.M.; Thuluvath, P.; et al. Serum levels of metabolites produced by intestinal microbes and lipid moieties independently associated with acute-on-chronic liver failure and death in patients with cirrhosis. Gastroenterology 2020, 159, 1715–1730.e12. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Rochell, S.J.; Applegate, T.J. Threonine, arginine, and glutamine: Influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 2018, 97, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Dong, Y.; Huang, J.; Liao, C.; Lei, J.; Wang, Y.; Lai, Y.; Bian, Y.; He, Y.; Sun, J.; et al. Exogenous L-arginine increases intestinal stem cell function through CD90+ stromal cells producing mTORC1-induced Wnt2b. Commun. Biol. 2020, 3, 611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lv, Z.; Li, H.; Guo, S.; Liu, D.; Guo, Y. Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Br. J. Nutr. 2017, 118, 321–332. [Google Scholar] [CrossRef]
- Bouilly, J.; Sonigo, C.; Auffret, J.; Gibori, G.; Binart, N. Prolactin signaling mechanisms in ovary. Mol. Cell Endocrinol. 2012, 356, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Skwarło-Sońta, K. Prolactin as an immunoregulatory hormone in mammals and birds. Immunol. Lett. 1992, 33, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, N.; Zhang, X.; Shahzad, K.; Qi, R.; Zhang, Z.; Lu, Z.; Lu, Y.; Yu, X.; Zafar, M.H.; et al. Mixed silage with Chinese cabbage waste enhances antioxidant ability by increasing ascorbate and aldarate metabolism through rumen Prevotellaceae UCG-004 in Hu sheep. Front. Microbiol. 2022, 13, 978940. [Google Scholar] [CrossRef]
- Wang, G.; Sun, S.; Wu, X.; Yang, S.; Wu, Y.; Zhao, J.; Zhang, H.; Chen, W. Intestinal environmental disorders associate with the tissue damages induced by perfluorooctane sulfonate exposure. Ecotoxicol. Environ. Saf. 2020, 197, 110590. [Google Scholar] [CrossRef]
- Jo, J.K.; Seo, S.H.; Park, S.E.; Kim, H.W.; Kim, E.J.; Kim, J.S.; Pyo, J.Y.; Cho, K.M.; Kwon, S.J.; Park, D.H.; et al. Gut microbiome and metabolome profiles associated with high-fat diet in mice. Metabolites 2021, 11, 874–884. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]



| Item | Control | FMF |
|---|---|---|
| Initial body weight (g) | 9.21 ± 0.46 | 9.55 ± 0.58 |
| Final body weight (g) | 478.27 ± 26.87 a | 562.33 ± 36.83 c |
| ADG (g/d) | 18.76 ± 1.07 a | 22.11 ± 1.46 b |
| Final carcass weight (g) | 404.13 ± 25.53 a | 495.53 ± 37.96 c |
| Final chest muscle weight (g) | 91.31 ± 13.35 a | 103.05 ± 9.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Li, X.; Ding, Z.; Zhang, H.; Lv, W.; Yang, C.; He, D.; Zhu, L. Enhancing Growth and Gut Health in Squabs: The Impact of Fermented Mixed Feed. Animals 2024, 14, 1411. https://doi.org/10.3390/ani14101411
Xiao C, Li X, Ding Z, Zhang H, Lv W, Yang C, He D, Zhu L. Enhancing Growth and Gut Health in Squabs: The Impact of Fermented Mixed Feed. Animals. 2024; 14(10):1411. https://doi.org/10.3390/ani14101411
Chicago/Turabian StyleXiao, Changfeng, Xin Li, Zhizhao Ding, Hongcai Zhang, Wenwei Lv, Changsuo Yang, Daqian He, and Lihui Zhu. 2024. "Enhancing Growth and Gut Health in Squabs: The Impact of Fermented Mixed Feed" Animals 14, no. 10: 1411. https://doi.org/10.3390/ani14101411





