Innovations in Electric Current-Assisted Sintering for SOFC: A Review of Advances in Flash Sintering and Ultrafast High-Temperature Sintering
Abstract
:1. Introduction
2. Flash Sintering
2.1. FS Process and Mechanism
- Nucleation of Frenkel pairs: The applied electric field can promote the nucleation of Frenkel pairs (vacancies and interstitials) with opposite charges (one is a hole; the other is an electron). These pairs, when stripped of their charge by the electric field, become electrically neutral. As a result, they can move freely within the grain, migrate into grain boundaries and pores under the influence of sintering pressure, and thus contribute to sintering. Concurrently, the movement of holes and electrons generates an electric current that further promotes sintering [55,56,57,58].
- The formation and rapid movement of oxygen vacancies: Under an electric field, the anodic reaction causes the formation of oxygen vacancies, which move rapidly from the anode to the cathode. The oxygen vacancies capture electrons, resulting in the partial reduction of the oxide and the transformation of the material into a mixed conductor with significantly enhanced conductivity [53,59,60,61].
2.2. The Application of FS in SOFCs
2.2.1. Electrolyte
2.2.2. Anode
2.2.3. Cathode
2.2.4. SOFC Multilayers
2.2.5. Interconnect Coating
- It accelerates the sintering process and lowers the sintering temperature, enabling ceramics to be processed together with metals or other materials;
- It suppresses grain growth and satisfies the required mechanical properties;
- It is a more straightforward and cost-effective alternative to conventional sintering.
3. UHS
3.1. UHS Process and Mechanism
- The passage of electric current through carbon cloth generates Joule heat, providing an ultra-high heating rate. This heat bypasses the low-temperature region, reducing particle coarsening and maintaining a high capillary driving force for sintering [43].
- High sintering temperatures and rapid heating rates result in small particles with molten surfaces, which exhibit characteristics of rapid migration. This promotes grain rearrangement and accelerates densification [125].
- The shorter processing time increases the number of defects, leading to a reduction in activation energy and an increase in conductivity, which in turn promotes sintering [126].
3.2. The Application of UHS in SOFCs
4. Current Progress and Other Applications
5. Challenges and Future Perspectives
- Process Control Precision: Achieving consistent and uniform outcomes with FS and UHS is complicated by the need for precise control over electric fields, temperature, and sintering atmosphere. This variability often results in non-uniform material properties, underscoring the need for advanced mechanistic insights and optimization strategies. Future research should aim to understand the physicochemical interactions and phase transformations during sintering, focusing on the dynamics of fast heating and cooling and the impact on material defects and microstructures under non-equilibrium conditions.
- Material Compatibility and Diversity: Both FS and UHS encounter limitations with specific ceramics and composites, necessitating broader exploration of materials that are amenable to fast sintering processes without sacrificing structural integrity. Addressing this challenge involves developing new ceramic composites and coatings optimized for these sintering technologies, with enhanced ionic conductivities, mechanical properties, and thermal stabilities.
- Scalability for Industrial Production: Scaling FS and UHS to industrial levels presents significant challenges, particularly in ensuring uniform treatment of larger or complex-shaped components and maintaining process stability. Investigating scalable sintering solutions that can adapt FS and UHS for large-scale manufacturing is crucial. Collaborative efforts between academia, industry, and equipment manufacturers are pivotal in this regard.
- 4.
- Specialized Sintering Equipment: The requirement for advanced sintering systems capable of managing ultrafast heating rates and achieving high temperatures contributes to the cost and complexity of FS and UHS. Future pathways include the integration of these sintering techniques with additive manufacturing technologies, which could revolutionize SOFC component design and fabrication by enabling complex geometries and tailored microstructures for enhanced performance.
- 5.
- Cost-Effectiveness and Energy Efficiency: The economic viability of FS and UHS in SOFC production hinges on balancing the initial investment in specialized equipment against the potential energy savings and improved efficiency from reduced sintering times. Emphasizing sustainable manufacturing practices that reduce the energy footprint of SOFC production aligns with global sustainability goals, urging further quantification of the environmental and economic impacts of these technologies.
- 6.
- Synergy with other processes and hybrid sintering techniques: Exploring FS and UHS in conjunction with other emerging sintering technologies could provide balanced solutions to existing challenges. This approach promises uniform material properties, reduced thermal gradients, and improved densification outcomes, marking a transformative era for SOFC fabrication.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges. Int. J. Hydrogen Energy 2012, 37, 449–470. [Google Scholar] [CrossRef]
- Deepi, A.S.; Dharani Priya, S.; Samson Nesaraj, A.; Selvakumar, A.I. Component fabrication techniques for solid oxide fuel cell (SOFC)—A comprehensive review and future prospects. Int. J. Green Energy 2022, 19, 1600–1612. [Google Scholar] [CrossRef]
- Dziurdzia, B.; Magonski, Z.; Jankowski, H. Commercialisation of Solid Oxide Fuel Cells—Opportunities and forecasts. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012020. [Google Scholar] [CrossRef]
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent advances on electrolyte materials for SOFC: A review. Inorg Chem Commun 2023, 152, 110724. [Google Scholar] [CrossRef]
- Mohd Affandi, N.S.; Osman, N. Short review on global trends in SOFC scenario and future perspective. Mater. Today Proc. 2022, 66, 3981–3984. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Kaleem Ullah, M.; Usman, A. A Brief Description of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Zhu, J.; Geng, S.; Ballard, D. Evaluation of several low thermal expansion Fe–Co–Ni alloys as interconnect for reduced-temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2007, 32, 3682–3688. [Google Scholar] [CrossRef]
- Gu, H.; Chen, H.; Gao, L.; Zheng, Y.; Zhu, X.; Guo, L. Effect of Co doping on the properties of Sr0.8Ce0.2MnO3−δ cathode for intermediate-temperature solid-oxide fuel cells. Int. J. Hydrogen Energy 2008, 33, 4681–4688. [Google Scholar] [CrossRef]
- Kamalimeera, N.; Kirubakaran, V. Prospects and restraints in biogas fed SOFC for rural energization: A critical review in indian perspective. Renew. Sustain. Energy Rev. 2021, 143, 110914. [Google Scholar] [CrossRef]
- Kostogloudis, G. Chemical reactivity of perovskite oxide SOFC cathodes and yttria stabilized zirconia. Solid State Ion. 2000, 135, 529–535. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef]
- Saebea, D.; Authayanun, S.; Patcharavorachot, Y.; Arpornwichanop, A. Performance Evaluation of Low-Temperature Solid Oxide Fuel Cells with SDC-Based Electrolyte. Chem. Eng. Trans. 2016, 52, 223–228. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Liu, Q. Development of LSCF–GDC composite cathodes for low-temperature solid oxide fuel cells with thin film GDC electrolyte. Int. J. Hydrogen Energy 2008, 33, 3808–3817. [Google Scholar] [CrossRef]
- Steele, B. Material science aspects of SOFC technology with special reference to anode development. Solid State Ion. 1990, 40–41, 388–393. [Google Scholar] [CrossRef]
- Choy, K.; Bai, W.; Charojrochkul, S.; Steele, B.C.H. The development of intermediate-temperature solid oxide fuel cells for the next millennium. J. Power Sources 1998, 71, 361–369. [Google Scholar] [CrossRef]
- Milliken, C.; Guruswamy, S.; Khandkar, A. Evaluation of Ceria Electrolytes in Solid Oxide Fuel Cells Electric Power Generation. J. Electrochem. Soc. 2019, 146, 872–882. [Google Scholar] [CrossRef]
- Maric, R.; Ohara, S.; Fukui, T.; Yoshida, H.; Nishimura, M.; Inagaki, T.; Miura, K. Solid Oxide Fuel Cells with Doped Lanthanum Gallate Electrolyte and LaSrCoO3 Cathode, and Ni-Samaria-Doped Ceria Cermet Anode. J. Electrochem. Soc. 2019, 146, 2006–2010. [Google Scholar] [CrossRef]
- Hibino, T.; Tsunekawa, H.; Tanimoto, S.; Sano, M. Improvement of a Single-Chamber Solid-Oxide Fuel Cell and Evaluation of New Cell Designs. J. Electrochem. Soc. 2000, 147, 1338. [Google Scholar] [CrossRef]
- Doshi, R.; Richards, V.L.; Carter, J.D.; Wang, X.; Krumpelt, M. Development of Solid-Oxide Fuel Cells That Operate at 500 °C. J. Electrochem. Soc. 2019, 146, 1273–1278. [Google Scholar] [CrossRef]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liu, J. An anode-supported solid oxide fuel cell with spray-coated yttria-stabilized zirconia (YSZ) electrolyte film. Solid State Ion. 2008, 179, 1246–1249. [Google Scholar] [CrossRef]
- Sındıraç, C.; Çakırlar, S.; Büyükaksoy, A.; Akkurt, S. Lowering the sintering temperature of solid oxide fuel cell electrolytes by infiltration. J. Eur. Ceram. Soc. 2019, 39, 409–417. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Pavitra, R.; Senthil Kumar, S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Maizza, G. Electric current activated/assisted sintering (ECAS): A review of patents 1906-2008. Sci. Technol. Adv. Mater. 2009, 10, 053001. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R. Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Nygren, M.; Shen, Z. On the preparation of bio-, nano- and structural ceramics and composites by spark plasma sintering. Solid State Sci. 2003, 5, 125–131. [Google Scholar] [CrossRef]
- Mamedov, V. Spark plasma sintering as advanced PM sintering method. Powder Metall. 2013, 45, 322–328. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Groza, J.R.; Zavaliangos, A. Sintering activation by external electrical field. Mater. Sci. Eng. A 2000, 287, 171–177. [Google Scholar] [CrossRef]
- Guillon, O.; Rheinheimer, W.; Bram, M. A Perspective on Emerging and Future Sintering Technologies of Ceramic Materials. Adv. Eng. Mater. 2023, 25, 2201870. [Google Scholar] [CrossRef]
- Demirskyi, D.; Vasylkiv, O. Hot-spots generation, exaggerated grain growth and mechanical performance of silicon carbide bulks consolidated by flash spark plasma sintering. J. Alloys Compd. 2017, 691, 466–473. [Google Scholar] [CrossRef]
- Xu, J.K.; Liu, Z.T.; Xie, Z.P.; He, S.; Xi, X.Q. DC electric field-assisted hot pressing of zirconia: Methodology, phenomenology, and sintering mechanism. J. Am. Ceram. Soc. 2021, 104, 5571–5583. [Google Scholar] [CrossRef]
- Alem, S.A.A.; Latifi, R.; Angizi, S.; Hassanaghaei, F.; Aghaahmadi, M.; Ghasali, E.; Rajabi, M. Microwave sintering of ceramic reinforced metal matrix composites and their properties: A review. Mater. Manuf. Process. 2020, 35, 303–327. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Kermani, M.; Hu, C.; Grasso, S. From pit fire to Ultrafast High-temperature Sintering (UHS): A review on ultrarapid consolidation. Ceram. Int. 2023, 49, 4017–4029. [Google Scholar] [CrossRef]
- Biesuz, M.; Grasso, S.; Sglavo, V.M. What’s new in ceramics sintering? A short report on the latest trends and future prospects. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Wang, R.H.; Yang, J.; Zou, D.N.; Hu, P.; Xiang, W.C. Recent Progress on Microwave Sintering of Metal Materials. Mater. Rep. 2021, 35, 23153–23161+23170. (In Chinese) [Google Scholar]
- Ye, F.X.; Meng, F.W.; Luo, T.Y.; Qi, H. Ultrafast high-temperature sintering of (Y0.2Dy0.2Er0.2Tm0.2Yb0.2)4Hf3O12 high-entropy ceramics with defective fluorite structure. J. Eur. Ceram. Soc. 2022, 42, 4686–4691. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash Sintering of Nanograin Zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef]
- Guo, R.-F.; Mao, H.-R.; Zhao, Z.-T.; Shen, P. Ultrafast high-temperature sintering of bulk oxides. Scr. Mater. 2021, 193, 103–107. [Google Scholar] [CrossRef]
- Kermani, M.; Dong, J.; Biesuz, M.; Linx, Y.; Deng, H.; Sglavo, V.M.; Reece, M.J.; Hu, C.; Grasso, S. Ultrafast high-temperature sintering (UHS) of fine grained α-Al2O3. J. Eur. Ceram. Soc. 2021, 41, 6626–6633. [Google Scholar] [CrossRef]
- Bhandari, S.; Manière, C.; Sedona, F.; De Bona, E.; Sglavo, V.M.; Colombo, P.; Fambri, L.; Biesuz, M.; Franchin, G. Ultra-rapid debinding and sintering of additively manufactured ceramics by ultrafast high-temperature sintering. J. Eur. Ceram. Soc. 2024, 44, 328–340. [Google Scholar] [CrossRef]
- Behera, R.P.; Reavley, M.J.-H.; Du, Z.; Gan, C.L.; Le Ferrand, H. Ultrafast high-temperature sintering of dense and textured alumina. J. Eur. Ceram. Soc. 2022, 42, 7122–7133. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Y.; Kermani, M.; Hu, C.; Grasso, S. Ultra-fast high temperature sintering (UHS) of Li1.5Al0.5Ge1.5P3O12 electrolyte: A rationalization of the heating schedule. Ceram. Int. 2022, 48, 6356–6362. [Google Scholar] [CrossRef]
- Dancer, C.E.J. Flash sintering of ceramic materials. Mater. Res. Express 2016, 3, 102001. [Google Scholar] [CrossRef]
- Mishra, R.R.; Sharma, A.K. Microwave–material interaction phenomena: Heating mechanisms, challenges and opportunities in material processing. Compos. Part A Appl. Sci. Manuf. 2016, 81, 78–97. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, Z.H.; Cheng, X.W.; Huo, G.J.; Zhang, S.Z.; Song, Q. The Development and Application of Spark Plasma Sintering Technique in Advanced Metal Structure Materials: A Review. Powder Metall. Met. Ceram. 2021, 60, 410–438. [Google Scholar] [CrossRef]
- Yu, M.; Grasso, S.; McKinnon, R.; Saunders, T.; Reece, M.J. Review of flash sintering: Materials, mechanisms and modelling. Adv. Appl. Ceram. 2016, 116, 24–60. [Google Scholar] [CrossRef]
- Biesuz, M.; Sglavo, V.M. Flash sintering of ceramics. J. Eur. Ceram. Soc. 2019, 39, 115–143. [Google Scholar] [CrossRef]
- Todd, R.I.; Zapata-Solvas, E.; Bonilla, R.S.; Sneddon, T.; Wilshaw, P.R. Electrical characteristics of flash sintering: Thermal runaway of Joule heating. J. Eur. Ceram. Soc. 2015, 35, 1865–1877. [Google Scholar] [CrossRef]
- Cologna, M.; Francis, J.S.C.; Raj, R. Field assisted and flash sintering of alumina and its relationship to conductivity and MgO-doping. J. Eur. Ceram. Soc. 2011, 31, 2827–2837. [Google Scholar] [CrossRef]
- Raj, R.; Cologna, M.; Francis, J.S.C. Influence of Externally Imposed and Internally Generated Electrical Fields on Grain Growth, Diffusional Creep, Sintering and Related Phenomena in Ceramics. J. Am. Ceram. Soc. 2011, 94, 1941–1965. [Google Scholar] [CrossRef]
- Raj, R. Joule heating during flash-sintering. J. Eur. Ceram. Soc. 2012, 32, 2293–2301. [Google Scholar] [CrossRef]
- Francis, J.S.C.; Raj, R. Flash-Sinterforging of Nanograin Zirconia: Field Assisted Sintering and Superplasticity. J. Am. Ceram. Soc. 2012, 95, 138–146. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Zhu, Y.; Liu, J.; Wu, A.; Ma, G.; Wang, X.; Jia, Z.; Wang, L. Review of flash sintering with strong electric field. High Volt. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Narayan, J. A new mechanism for field-assisted processing and flash sintering of materials. Scr. Mater. 2013, 69, 107–111. [Google Scholar] [CrossRef]
- Janek, J.; Korte, C. Electrochemical blackening of yttria-stabilized zirconia—Morphological instability of the moving reaction front. Solid State Ion. 1999, 116, 181–195. [Google Scholar] [CrossRef]
- Brandner, M.; Bram, M.; Froitzheim, J.; Buchkremer, H.P.; Stöver, D. Electrically Conductive Diffusion barrier layers for Metal-Supported SOFC. Solid State Ion. 2008, 179, 1501–1504. [Google Scholar] [CrossRef]
- Francis, J.S.C.; Raj, R.; Halloran, J. Influence of the Field and the Current Limit on Flash Sintering at Isothermal Furnace Temperatures. J. Am. Ceram. Soc. 2013, 96, 2754–2758. [Google Scholar] [CrossRef]
- Naik, K.S.; Sglavo, V.M.; Raj, R. Flash sintering as a nucleation phenomenon and a model thereof. J. Eur. Ceram. Soc. 2014, 34, 4063–4067. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Biesuz, M.; Dell’Agli, G.; Di Bartolomeo, E.; Zurlo, F.; Sglavo, V.M. Microstructural and electrical investigation of flash-sintered Gd/Sm-doped ceria. J. Mater. Sci. 2017, 52, 7479–7488. [Google Scholar] [CrossRef]
- Li, J.; Guan, L.; Zhang, W.; Luo, M.; Song, J.; Song, X.; An, S. Sintering behavior of samarium doped ceria under DC electrical field. Ceram. Int. 2018, 44, 2470–2477. [Google Scholar] [CrossRef]
- Mohammad Alizadeh, S.; Mirkazemi, S.M.; Mohebbi, H. Prolonged flash sintering and its effects on defect chemistry, phase transformation and ionic conductivity of yttria-stabilized zirconia. Appl. Phys. A 2022, 128, 804. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Zhang, J.; Hao, X.; Rooney, D.; Liu, Y.; Sun, W.; Qiao, J.; Sun, K.; Hay, R. Understanding the Flash Sintering of Rare-Earth-Doped Ceria for Solid Oxide Fuel Cell. J. Am. Ceram. Soc. 2015, 98, 1717–1723. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, J.; Jiang, T.; Qiao, J.; Sun, W.; Rooney, D.; Wang, Z. Flash-Sintering and Characterization of La0.8Sr0.2Ga0.8Mg0.2O3-δ Electrolytes for Solid Oxide Fuel Cells. Electrochim. Acta 2016, 196, 487–495. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, Y.; Wang, Z.; Sun, W.; Qiao, J.; Sun, K. An improved direct current sintering technique for proton conductor—BaZr0.1Ce0.7Y0.1Yb0.1O3: The effect of direct current on sintering process. J. Power Sources 2014, 248, 70–76. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced Sulfur and Coking Tolerance of a Mixed Ion Conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 2009, 326, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Cologna, M.; Prette, A.L.G.; Raj, R. Flash-Sintering of Cubic Yttria-Stabilized Zirconia at 750 °C for Possible Use in SOFC Manufacturing. J. Am. Ceram. Soc. 2011, 94, 316–319. [Google Scholar] [CrossRef]
- M’Peko, J.C.; Francis, J.S.C.; Raj, R. Impedance Spectroscopy and Dielectric Properties of Flash Versus Conventionally Sintered Yttria-Doped Zirconia Electroceramics Viewed at the Microstructural Level. J. Am. Ceram. Soc. 2013, 96, 3760–3767. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Gao, Y.; Liu, F.; Li, K.; Xia, J.; Wang, Y.; An, L. Effect of the applied electric field on the microstructure and electrical properties of flash-sintered 3YSZ ceramics. Ceram. Int. 2016, 42, 19066–19070. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Jiang, T.; Xie, L.; Sui, C.; Ren, R.; Qiao, J.; Sun, K. Densification of 8 mol% yttria-stabilized zirconia at low temperature by flash sintering technique for solid oxide fuel cells. Ceram. Int. 2017, 43, 14037–14043. [Google Scholar] [CrossRef]
- Mohebbi, H.; Mirkazemi, S.M. Influence of electric field strength on structure, microstructure, and electrical properties of flash sintered 8% mol Yttria-stabilized zirconia as a solid oxide fuel cell electrolyte. Ceram. Int. 2021, 47, 20220–20229. [Google Scholar] [CrossRef]
- Hao, X.; Liu, Y.; Wang, Z.; Qiao, J.; Sun, K. A novel sintering method to obtain fully dense gadolinia doped ceria by applying a direct current. J. Power Sources 2012, 210, 86–91. [Google Scholar] [CrossRef]
- Cologna, M.; Raj, R. Surface Diffusion-Controlled Neck Growth Kinetics in Early Stage Sintering of Zirconia, with and without Applied DC Electrical Field. J. Am. Ceram. Soc. 2010, 94, 391–395. [Google Scholar] [CrossRef]
- Valdebenito, J.U.; Akbari-Fakhrabadi, A.; Viswanathan, M.R. Effect of flash sintering on microstructure of Ce0.9Gd0.1O1.95 electrolyte fabricated by tape-casting. Mater. Lett. 2017, 209, 291–294. [Google Scholar] [CrossRef]
- Mishra, T.P.; Neto, R.R.I.; Speranza, G.; Quaranta, A.; Sglavo, V.M.; Raj, R.; Guillon, O.; Bram, M.; Biesuz, M. Electronic conductivity in gadolinium doped ceria under direct current as a trigger for flash sintering. Scr. Mater. 2020, 179, 55–60. [Google Scholar] [CrossRef]
- Huijsmans, J.P.P.; van Berkel, F.P.F.; Christie, G.M. Intermediate temperature SOFC—A promise for the 21st century. J. Power Sources 1998, 71, 107–110. [Google Scholar] [CrossRef]
- Ishiyama, T.; Kishimoto, H.; Develos-Bagarinao, K.; Yamaji, K.; Yamaguchi, T.; Fujishiro, Y. Correlation between Dissolved Protons in Nickel-Doped BaZr0.1Ce0.7Y0.1Yb0.1O3−δ and Its Electrical Conductive Properties. Inorg. Chem. 2017, 56, 11876–11882. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takita, Y. Doped LaGaO3 Perovskite Type Oxide as a New Oxide Ionic Conductor. J. Am. Chem. Soc. 2002, 116, 3801–3803. [Google Scholar] [CrossRef]
- Yang, N.; D’Epifanio, A.; Di Bartolomeo, E.; Pugnalini, C.; Tebano, A.; Balestrino, G.; Licoccia, S. La0.8Sr0.2Ga0.8Mg0.2O3−δ thin films for IT-SOFCs: Microstructure and transport properties correlation. J. Power Sources 2013, 222, 10–14. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Qiao, J.; Sun, W.; Sun, K.; Wang, Z. An easily controllable flash sintering process for densification of electrolyte for application in solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 17824–17832. [Google Scholar] [CrossRef]
- Bhandari, S.; Mishra, T.P.; Bram, M.; Guillon, O.; Yadav, D. Flash sintering behaviour of 8YSZ-NiO composites. Ceram. Int. 2022, 48, 33236–33244. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Jiang, S.; Khor, K. Low-temperature SOFC with thin film GDC electrolyte prepared in situ by solid-state reaction. Solid State Ion. 2004, 170, 9–15. [Google Scholar] [CrossRef]
- Hui, S.; Roller, J.; Yick, S.; Zhang, X.; Decès-Petit, C.; Xie, Y.; Maric, R.; Ghosh, D. A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J. Power Sources 2007, 172, 493–502. [Google Scholar] [CrossRef]
- Usuba, J.B.; Araújo, A.J.M.; Gonçalves, E.D.; Macedo, D.A.; Salvo, C.; Viswanathan, M.R. Flash sintering of one-step synthesized NiO-CeGdO (NiO-GDC) composite. Mater. Res. Express 2019, 6, 125535. [Google Scholar] [CrossRef]
- Menzler, N.H.; Tietz, F.; Uhlenbruck, S.; Buchkremer, H.P.; Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J. Mater. Sci. 2010, 45, 3109–3135. [Google Scholar] [CrossRef]
- Gaur, A.; Sglavo, V.M. Densification of La0.6Sr0.4Co0.2Fe0.8O3 ceramic by flash sintering at temperature less than 100 °C. J. Mater. Sci. 2014, 49, 6321–6332. [Google Scholar] [CrossRef]
- Gaur, A.; Sglavo, V.M.; Olevsky, E. Flash Sintering of (La, Sr)(Co, Fe)O3–Gd-Doped CeO2 Composite. J. Am. Ceram. Soc. 2015, 98, 1747–1752. [Google Scholar] [CrossRef]
- Accardo, G.; Kim, G.S.; Ham, H.C.; Yoon, S.P. Optimized lithium-doped ceramic electrolytes and their use in fabrication of an electrolyte-supported solid oxide fuel cell. Int. J. Hydrogen Energy 2019, 44, 12138–12150. [Google Scholar] [CrossRef]
- Bi, L.; Tao, Z.; Sun, W.; Zhang, S.; Peng, R.; Liu, W. Proton-conducting solid oxide fuel cells prepared by a single step co-firing process. J. Power Sources 2009, 191, 428–432. [Google Scholar] [CrossRef]
- Jin, C.; Mao, Y.; Zhang, N.; Sun, K. Fabrication and characterization of Ni-SSZ/SSZ/LSM-SSZ anode-supported SOFCs by tape casting and single-step co-sintering techniques. Ionics 2016, 22, 1145–1152. [Google Scholar] [CrossRef]
- Dai, H.; Chen, H.; He, S.; Cai, G.; Guo, L. Improving solid oxide fuel cell performance by a single-step co-firing process. J. Power Sources 2015, 286, 427–430. [Google Scholar] [CrossRef]
- Han, M.-F.; Yin, H.-Y.; Miao, W.-T.; Zhou, S. Fabrication and properties of anode-supported solid oxide fuel cell. Solid State Ion. 2008, 179, 1545–1548. [Google Scholar] [CrossRef]
- Moon, H.; Kim, S.; Hyun, S.; Kim, H. Development of IT-SOFC unit cells with anode-supported thin electrolytes via tape casting and co-firing. Int. J. Hydrogen Energy 2008, 33, 1758–1768. [Google Scholar] [CrossRef]
- Yoon, K.J.; Zink, P.; Gopalan, S.; Pal, U.B. Polarization measurements on single-step co-fired solid oxide fuel cells (SOFCs). J. Power Sources 2007, 172, 39–49. [Google Scholar] [CrossRef]
- Gladysz, G.M.; Chawla, K.K. Coefficients of thermal expansion of some laminated ceramic composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 173–178. [Google Scholar] [CrossRef]
- Alemayehu, A.; Biesuz, M.; Javan, K.Y.; Tkach, A.; Vilarinho, P.M.; Sglavo, V.M.; Tyrpekl, V. Ultrafast high-temperature sintering of gadolinia-doped ceria. J. Eur. Ceram. Soc. 2023, 43, 4837–4843. [Google Scholar] [CrossRef]
- Francis, J.S.C.; Cologna, M.; Montinaro, D.; Raj, R.; Wei, W.C. Flash Sintering of Anode-Electrolyte Multilayers for SOFC Applications. J. Am. Ceram. Soc. 2013, 96, 1352–1354. [Google Scholar] [CrossRef]
- Jha, S.K.; Raj, R.; Hsueh, C.H. Electric Fields Obviate Constrained Sintering. J. Am. Ceram. Soc. 2014, 97, 3103–3109. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, X.; Wang, Z.; Wang, J.; Qiao, J.; Yan, Y.; Sun, K. A newly-developed effective direct current assisted sintering technique for electrolyte film densification of anode-supported solid oxide fuel cells. J. Power Sources 2012, 215, 296–300. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Liu, J.; Barnett, S. Direct operation of solid oxide fuel cells with methane fuel. Solid State Ion. 2005, 176, 1827–1835. [Google Scholar] [CrossRef]
- Liu, J.; Birss, V.; Hill, J. Electrochemical performance and microstructure characterization of nickel yttrium-stabilized zirconia anode. AIChE J. 2009, 56, 1651–1658. [Google Scholar] [CrossRef]
- Hao, X.; Han, D.; Wang, J.; Liu, Y.; Rooney, D.; Sun, W.; Qiao, J.; Wang, Z.; Sun, K. Co-tape casting fabrication, field assistant sintering and evaluation of a coke resistant La0.2Sr0.7TiO3–Ni/YSZ functional gradient anode supported solid oxide fuel cell. Int. J. Hydrogen Energy 2015, 40, 12790–12797. [Google Scholar] [CrossRef]
- Muccillo, R.; de Florio, D.Z.; Fonseca, F.C.; Carvalho, S.G.M.; Muccillo, E.N.S. Electric field-assisted sintering anode-supported single solid oxide fuel cell. Int. J. Appl. Ceram. Technol. 2021, 19, 906–912. [Google Scholar] [CrossRef]
- Ni, N.; Xiao, W.; Zheng, C.; Jiang, J.; Yu, Y.; Hao, W.; Tang, M.; Shen, H.; Peng, D. Flash cosintering of a lanthanum strontium cobalt ferrite nanofibre/Gd-doped ceria bilayer structure. J. Eur. Ceram. Soc. 2022, 42, 2870–2878. [Google Scholar] [CrossRef]
- Petric, A.; Ling, H. Electrical Conductivity and Thermal Expansion of Spinels at Elevated Temperatures. J. Am. Ceram. Soc. 2007, 90, 1515–1520. [Google Scholar] [CrossRef]
- Wang, K.L.; Liu, Y.J.; Fergus, J.W. Interactions Between SOFC Interconnect Coating Materials and Chromia. J. Am. Ceram. Soc. 2011, 94, 4490–4495. [Google Scholar] [CrossRef]
- Prette, A.L.G.; Cologna, M.; Sglavo, V.; Raj, R. Flash-sintering of Co2MnO4 spinel for solid oxide fuel cell applications. J. Power Sources 2011, 196, 2061–2065. [Google Scholar] [CrossRef]
- Gaur, A.; Sglavo, V.M. Flash-sintering of MnCo2O4 and its relation to phase stability. J. Eur. Ceram. Soc. 2014, 34, 2391–2400. [Google Scholar] [CrossRef]
- Jones, G.M.; Biesuz, M.; Ji, W.; John, S.F.; Grimley, C.; Manière, C.; Dancer, C.E.J. Promoting microstructural homogeneity during flash sintering of ceramics through thermal management. MRS Bull. 2021, 46, 59–66. [Google Scholar] [CrossRef]
- Dong, Y. On the Hotspot Problem in Flash Sintering. arXiv 2017, arXiv:1702.05565. [Google Scholar]
- Raj, R.; Wolf, D.E.; Yamada, C.N.; Jha, S.K.; Lebrun, J.M. On the confluence of ultrafast high-temperature sintering and flash sintering phenomena. J. Am. Ceram. Soc. 2023, 106, 3983–3998. [Google Scholar] [CrossRef]
- Bram, M.; Laptev, A.M.; Mishra, T.P.; Nur, K.; Kindelmann, M.; Ihrig, M.; Pereira da Silva, J.G.; Steinert, R.; Buchkremer, H.P.; Litnovsky, A.; et al. Application of Electric Current-Assisted Sintering Techniques for the Processing of Advanced Materials. Adv. Eng. Mater. 2020, 22, 2000051. [Google Scholar] [CrossRef]
- Saunders, T.; Grasso, S.; Reece, M.J. Ultrafast-Contactless Flash Sintering using Plasma Electrodes. Sci. Rep. 2016, 6, 27222. [Google Scholar] [CrossRef]
- Xiong, G.; Jia, J.; Zhao, L.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Non-thermal radiation heating synthesis of nanomaterials. Sci. Bull. 2021, 66, 386–406. [Google Scholar] [CrossRef]
- Lyu, Z.; Chen, R.; Mavrikakis, M.; Xia, Y. Physical Transformations of Noble-Metal Nanocrystals upon Thermal Activation. Acc. Chem. Res. 2021, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, X.; Han, Y.-C.; Chen, Y.; Hu, W. High-temperature shock synthesis of high-entropy-alloy nanoparticles for catalysis. Chin. J. Catal. 2023, 48, 66–89. [Google Scholar] [CrossRef]
- Dou, S.; Xu, J.; Cui, X.; Liu, W.; Zhang, Z.; Deng, Y.; Hu, W.; Chen, Y. High-Temperature Shock Enabled Nanomanufacturing for Energy-Related Applications. Adv. Energy Mater. 2020, 10, 2001331. [Google Scholar] [CrossRef]
- Chen, Y.; Egan, G.C.; Wan, J.; Zhu, S.; Jacob, R.J.; Zhou, W.; Dai, J.; Wang, Y.; Danner, V.A.; Yao, Y.; et al. Ultra-fast self-assembly and stabilization of reactive nanoparticles in reduced graphene oxide films. Nat. Commun. 2016, 7, 12332. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Xu, C.; Fu, Z.; Zhang, J. Densification mechanism of the ultra-fast sintering dense alumina. AIP Adv. 2020, 10, 025223. [Google Scholar] [CrossRef]
- Li, Q.; Du, H.; Zhao, X.; Zhao, F.; Zhang, Y.; Hu, X.; Du, X. Densification and microstructure evolution of NaNbO3 ceramic via ultrafast high-temperature sintering. Ceram. Int. 2024, 50, 18907–18914. [Google Scholar] [CrossRef]
- Dong, J.; Pouchly, V.; Biesuz, M.; Tyrpekl, V.; Vilémová, M.; Kermani, M.; Reece, M.; Hu, C.; Grasso, S. Thermally-insulated ultra-fast high temperature sintering (UHS) of zirconia: A master sintering curve analysis. Scr. Mater. 2021, 203, 114076. [Google Scholar] [CrossRef]
- Wang, S.F.; Mishra, T.P.; Deng, Y.B.; Balice, L.; Kaletsch, A.; Bram, M.; Broeckmann, C. Electric Current-Assisted Sintering of 8YSZ: A Comparative Study of Ultrafast High-Temperature Sintering and Flash Sintering. Adv. Eng. Mater. 2023, 25, 2300145. [Google Scholar] [CrossRef]
- Wu, J.; Kermani, M.; Zhu, D.; Li, J.; Lin, Y.; Hu, C.; Grasso, S. Carbon free ultra-fast high temperature sintering of translucent zirconia. Scr. Mater. 2022, 210, 114476. [Google Scholar] [CrossRef]
- Biesuz, M.; Beauvoir, T.H.d.; De Bona, E.; Cassetta, M.; Manière, C.; Sglavo, V.M.; Estournès, C. Ultrafast high-temperature sintering (UHS) vs. conventional sintering of 3YSZ: Microstructure and properties. J. Eur. Ceram. Soc. 2024, 44, 4741–4750. [Google Scholar] [CrossRef]
- Guo, M.; Dong, Q.; Xie, H.; Wang, C.; Zhao, Y.; Wang, X.; Zhong, W.; Li, Z.; Wang, R.; Wang, Y.; et al. Ultrafast high-temperature sintering to avoid metal loss toward high-performance and scalable cermets. Matter 2022, 5, 594–604. [Google Scholar] [CrossRef]
- Guo, R.-F.; Zhao, Z.-T.; Shen, P. Ultrafast high-temperature sintering of lanthanum-chromite-based ceramics. J. Eur. Ceram. Soc. 2022, 42, 7072–7080. [Google Scholar] [CrossRef]
- Usseinov, A.B.; Karipbayev, Z.T.; Purans, J.; Kakimov, A.B.; Bakytkyzy, A.; Zhunusbekov, A.M.; Koketai, T.A.; Kozlovskyi, A.L.; Suchikova, Y.; Popov, A.I. Study of β-Ga2O3 Ceramics Synthesized under Powerful Electron Beam. Materials 2023, 16, 6997. [Google Scholar] [CrossRef] [PubMed]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.I.; Polisadova, E.F.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-resolved luminescence of YAG:Ce and YAGG:Ce ceramics prepared by electron beam assisted synthesis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Dahl, P.; Kaus, I.; Zhao, Z.; Johnsson, M.; Nygren, M.; Wiik, K.; Grande, T.; Einarsrud, M.A. Densification and properties of zirconia prepared by three different sintering techniques. Ceram. Int. 2007, 33, 1603–1610. [Google Scholar] [CrossRef]
- Razavi Hesabi, Z.; Mazaheri, M.; Ebadzadeh, T. Enhanced electrical conductivity of ultrafine-grained 8Y2O3 stabilized ZrO2 produced by two-step sintering technique. J. Alloys Compd. 2010, 494, 362–365. [Google Scholar] [CrossRef]
- Joh, D.W.; Rath, M.K.; Park, J.W.; Park, J.H.; Cho, K.H.; Lee, S.; Yoon, K.J.; Lee, J.-H.; Lee, K.T. Sintering behavior and electrochemical performances of nano-sized gadolinium-doped ceria via ammonium carbonate assisted co-precipitation for solid oxide fuel cells. J. Alloys Compd. 2016, 682, 188–195. [Google Scholar] [CrossRef]
- Kabir, A.; Colding-Jørgensen, S.; Molin, S.; Esposito, V. Electrical conductivity of nanostructured acceptor-doped ceria fabricated by spark plasma sintering (SPS). Mater. Lett. 2020, 279, 128513. [Google Scholar] [CrossRef]
- Egorov, S.V.; Eremeev, A.G.; Kholoptsev, V.V.; Plotnikov, I.V.; Rybakov, K.I.; Sorokin, A.A.; Balabanov, S.S.; Rostokina, E.Y. Rapid microwave sintering of gadolinia-doped ceria. Materialia 2024, 33, 101980. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, Q.; Yan, Z.-Q.; Kermani, M.; Grasso, S.; Nie, G.-L.; Jiang, B.-B.; He, F.-P.; Lin, H.-T.; Wang, L.-G. Upscaling Ultrafast High-Temperature Sintering (UHS) to consolidate large-sized and complex-shaped ceramics. Scr. Mater. 2022, 221, 114973. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Z.; Zhao, X.; Biesuz, M.; Saunders, T.; Zhang, Z.; Hu, C.; Grasso, S. Contactless flash sintering based on cold plasma. Scr. Mater. 2020, 175, 20–23. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Chan, J.M.; Huang, R.; Luo, J. Water-assisted flash sintering: Flashing ZnO at room temperature to achieve ~98% density in seconds. Scr. Mater. 2018, 142, 79–82. [Google Scholar] [CrossRef]
- Hérisson de Beauvoir, T.; Ghomari, Z.; Chevallier, G.; Flaureau, A.; Weibel, A.; Elissalde, C.; Mauvy, F.; Chaim, R.; Estournès, C. Flash Spark Plasma Sintering of 3YSZ: Modified sintering pathway and impact on grain boundary formation. J. Eur. Ceram. Soc. 2021, 41, 7762–7770. [Google Scholar] [CrossRef]
- Shen, H.-Z.; Guo, R.-F.; Shen, P. Efficient synthesis and densification of MgAl2O4 ceramics: Combining reactive cold sintering with ultrafast high-temperature sintering. J. Eur. Ceram. Soc. 2024, 44, 4816–4821. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, N.; Quattrocchi, E.; Ciucci, F.; Wu, J.; Kermani, M.; Dong, J.; Hu, C.; Grasso, S. Ultrafast high-temperature sintering (UHS) of Li1.3Al0.3Ti1.7(PO4)3. Ceram. Int. 2021, 47, 21982–21987. [Google Scholar] [CrossRef]
- Curcio, A.; Sabato, A.G.; Nuñez Eroles, M.; Gonzalez-Rosillo, J.C.; Morata, A.; Tarancón, A.; Ciucci, F. Ultrafast Crystallization and Sintering of Li1.5Al0.5Ge1.5(PO4)3 Glass and Its Impact on Ion Conduction. ACS Appl. Energy Mater. 2022, 5, 14466–14475. [Google Scholar] [CrossRef]
- Wu, C.; Fu, Y.; Zeng, Y.; Chen, G.; Pan, X.; Lin, F.; Xu, L.; Chen, Q.; Sun, D.; Hai, Z. Ultrafast high-temperature sintering of polymer-derived ceramic nanocomposites for high-temperature thin-film sensors. Chem. Eng. J. 2023, 463, 142518. [Google Scholar] [CrossRef]
- Ye, F.; Luo, T.; Meng, F.; Guo, L. Structure, thermal and mechanical properties of mid-entropy thermal barrier ceramic (Y0.3Gd0.3Yb0.4)4Hf3O12 prepared by ultrafast high-temperature sintering. Ceram. Int. 2024, 50, 181–187. [Google Scholar] [CrossRef]
- Zhao, Z.-T.; Guo, R.-F.; Mao, H.-R.; Shen, P. Effect of components on the microstructures and properties of rare-earth zirconate ceramics prepared by ultrafast high-throughput sintering. J. Eur. Ceram. Soc. 2021, 41, 5768–5773. [Google Scholar] [CrossRef]
- Boldrini, S.; Ferrario, A.; Fasolin, S.; Miozzo, A.; Barison, S. Ultrafast high-temperature sintering and thermoelectric properties of n-doped Mg2Si. Nanotechnology 2023, 34, 155601. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, W.; Ping, W.; Lin, Z.; Wang, R.; Dai, J.; Guo, M.; Xiong, W.; Zhao, J.-C.; Hu, L. Rapid Synthesis and Sintering of Metals from Powders. Adv. Sci. 2021, 8, 2004229. [Google Scholar] [CrossRef] [PubMed]
- Biesuz, M.; Galotta, A.; Motta, A.; Kermani, M.; Grasso, S.; Vontorová, J.; Tyrpekl, V.; Vilémová, M.; Sglavo, V.M. Speedy bioceramics: Rapid densification of tricalcium phosphate by ultrafast high-temperature sintering. Mater. Sci. Eng. C 2021, 127, 112246. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, X.; Wang, C.; Dong, Q.; Qian, J.; Zhang, G.; Brozena, A.H.; Wang, X.; He, S.; Ping, W.; et al. Rapid Pressureless Sintering of Glasses. Small 2022, 18, 2107951. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cao, Y.; Chen, Y.; Shao, G.; Dai, J.; Wang, Y. Flash sintering of Na3Zr2(SiO4)2(PO4) solid-state electrolyte at furnace temperature of 700 °C. Scr. Mater. 2020, 187, 384–389. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Bonilla, S.; Wilshaw, P.R.; Todd, R.I. Preliminary investigation of flash sintering of SiC. J. Eur. Ceram. Soc. 2013, 33, 2811–2816. [Google Scholar] [CrossRef]
- Gibson, A.; Li, Y.; Bonilla, R.S.; Todd, R.I. Pressureless flash sintering of α-SiC: Electrical characteristics and densification. Acta Mater. 2022, 241, 118362. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Flash sintering of tricalcium phosphate (TCP) bioceramics. J. Eur. Ceram. Soc. 2018, 38, 279–285. [Google Scholar] [CrossRef]
- Frasnelli, M.; Pedranz, A.; Biesuz, M.; Dirè, S.; Sglavo, V.M. Flash sintering of Mg-doped tricalcium phosphate (TCP) nanopowders. J. Eur. Ceram. Soc. 2019, 39, 3883–3892. [Google Scholar] [CrossRef]
- Xia, J.; Ren, K.; Wang, Y.; An, L. Reversible flash-bonding of zirconia and nickel alloys. Scr. Mater. 2018, 153, 31–34. [Google Scholar] [CrossRef]
- Xia, J.; Ren, K.; Wang, Y. Reversible joining of zirconia to titanium alloy. Ceram. Int. 2019, 45, 2509–2515. [Google Scholar] [CrossRef]
- Valdez, J.A.; Byler, D.D.; Kardoulaki, E.; Francis, J.S.C.; McClellan, K.J. Flash sintering of stoichiometric and hyper-stoichiometric urania. J. Nucl. Mater. 2018, 505, 85–93. [Google Scholar] [CrossRef]
- Raftery, A.M.; Pereira da Silva, J.G.; Byler, D.D.; Andersson, D.A.; Uberuaga, B.P.; Stanek, C.R.; McClellan, K.J. Onset conditions for flash sintering of UO2. J. Nucl. Mater. 2017, 493, 264–270. [Google Scholar] [CrossRef]
- Ingraci Neto, R.R.; McClellan, K.J.; Byler, D.D.; Kardoulaki, E. Controlled current-rate AC flash sintering of uranium dioxide. J. Nucl. Mater. 2021, 547, 152780. [Google Scholar] [CrossRef]

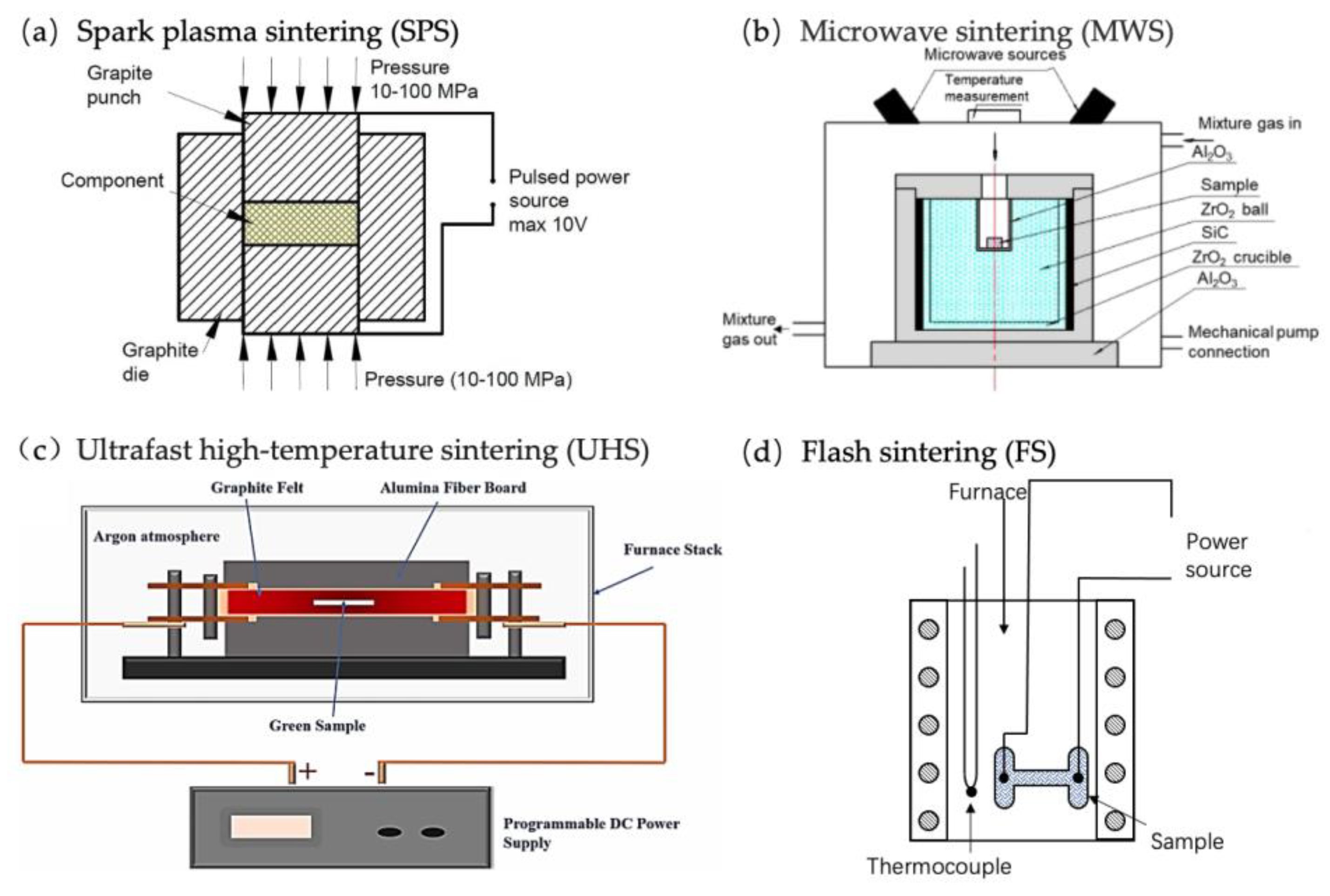

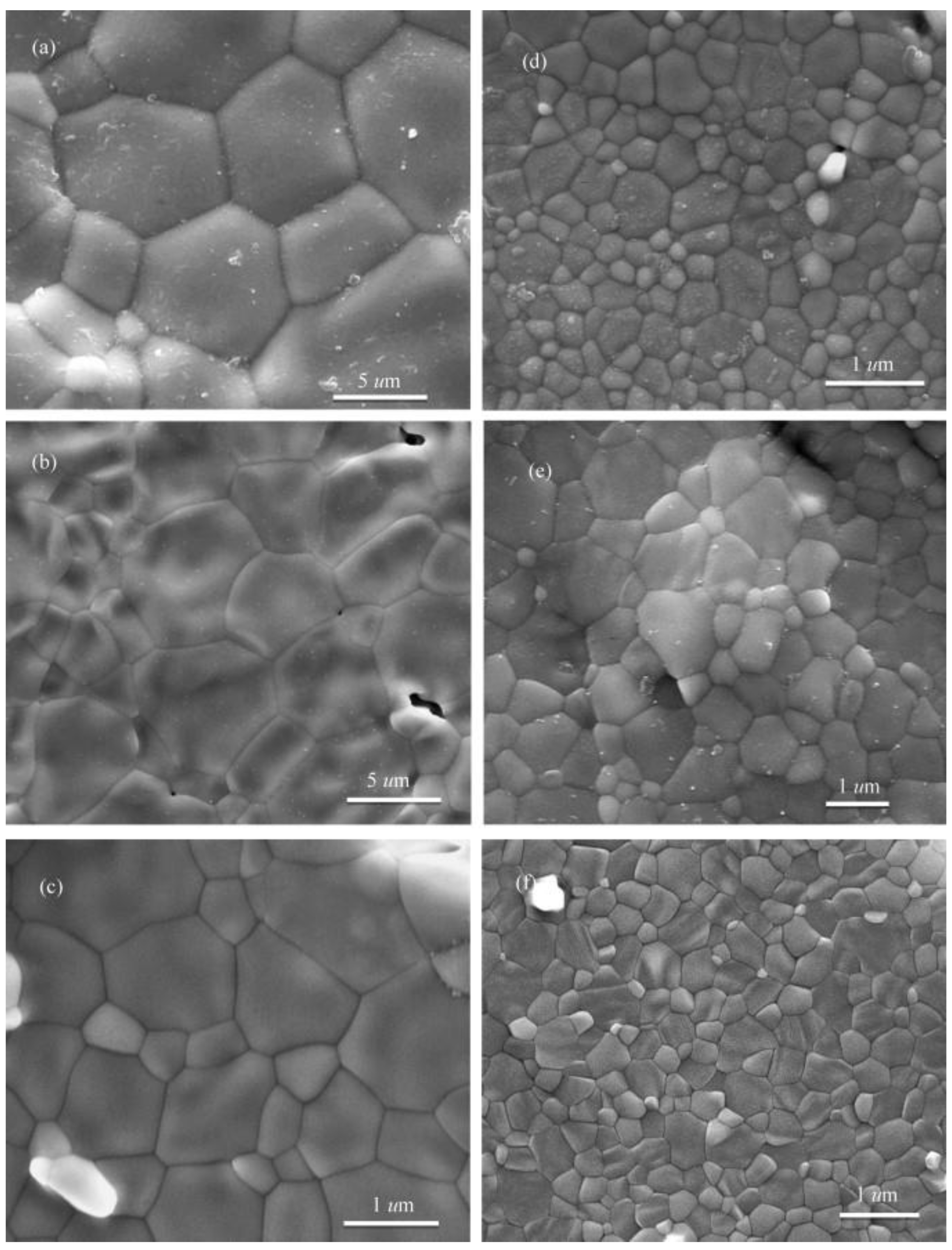

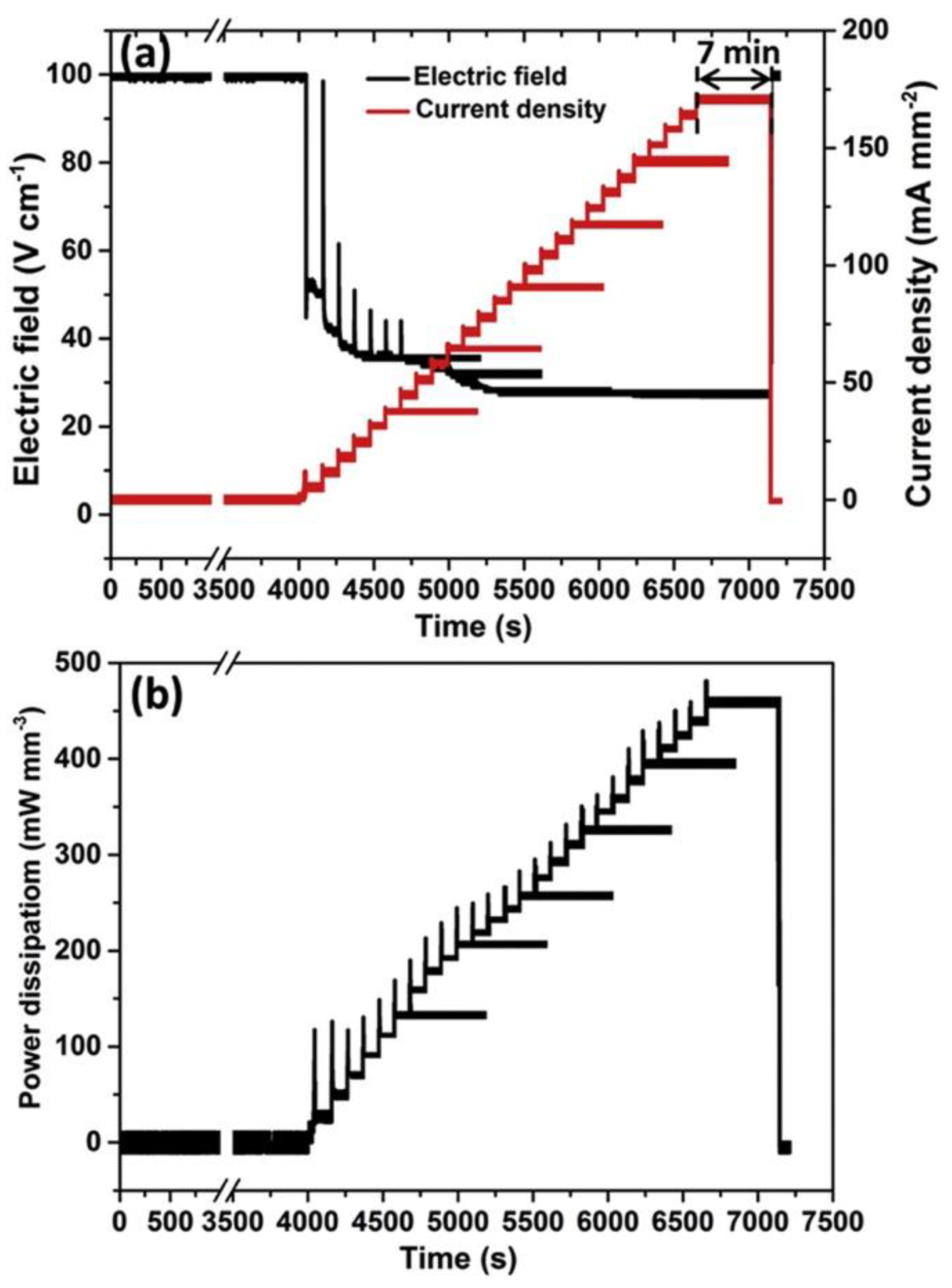

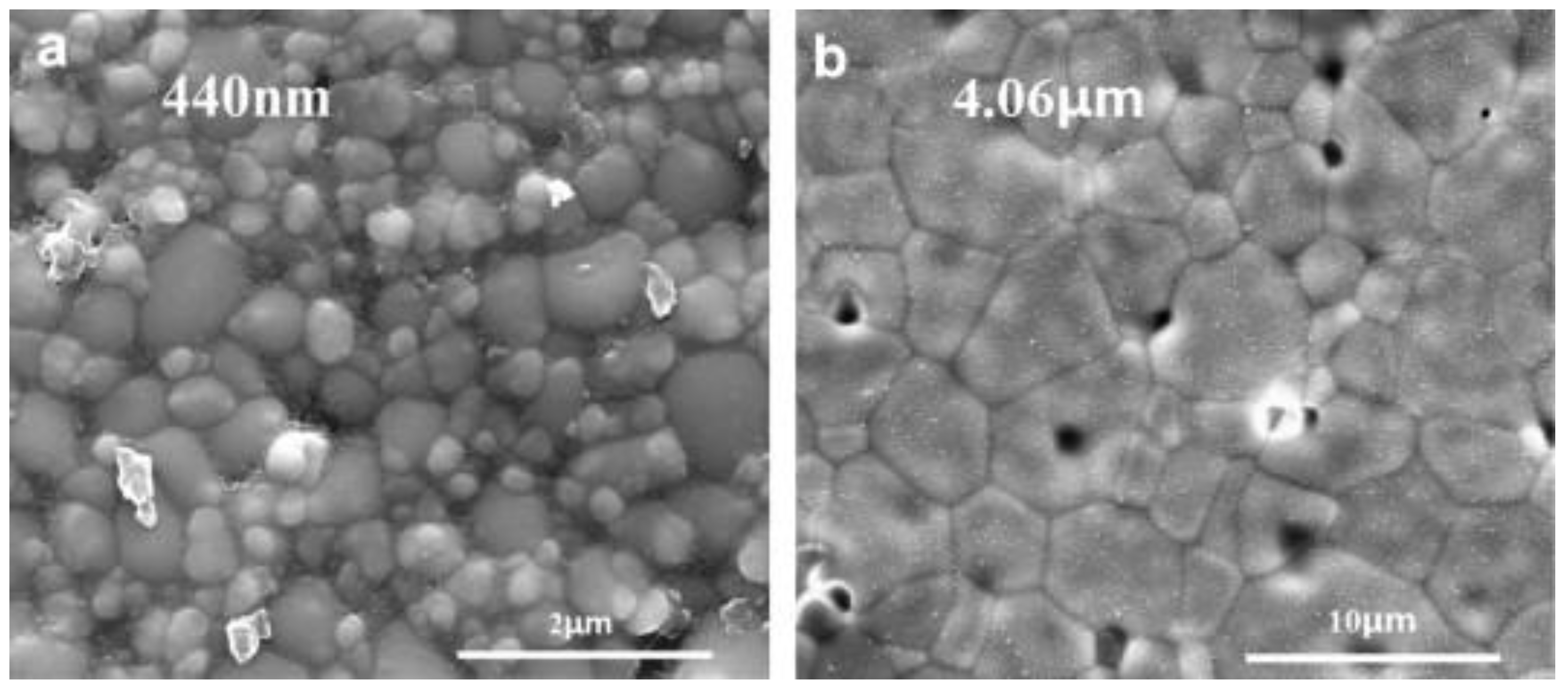








| Sintering Technique | Major Mechanism | Typical Heating Rate (°C min−1) | Typical Dwelling Time (min) | Typical Process Advantages | Major Limitations | References |
|---|---|---|---|---|---|---|
| CS | The furnace transfers heat to the sample through radiation, conduction, and convection. | 1–10 | >120 | Simple equipment, with no restrictions on sample size and shape. | Time-consuming and energy-consuming. Samples exhibit coarse grains. Restricted low melting point materials that can be co-processed with the ceramic. | [39,49] |
| MWS | The materials absorb microwave energy to provide volumetric heating. | 100 | <10 | Uniform heating, low energy consumption, the ability to sinter complex-shaped samples. Samples exhibit uniform microstructure, high density, and excellent mechanical properties. | The equipment is complex, and the process requires microwave absorption properties of the materials or uses susceptors. Utilizing the dielectric loss of the material to heat the sample. | [36,37,50] |
| SPS | The electric current generates Joule heating within the sample. | 102–103 | <20 | Low sintering temperature, fast speed, and high efficiency. Samples exhibit high density and fine grain size. | The equipment is complex and expensive, pressure-assisted, and has limitations in part dimension and geometries. | [31,51] |
| FS | The electric current generates Joule heating within the sample. Promotion of the ion diffusion depending on the material system. | 103–104 | <1 | Fast sintering speed, fine-grained samples. | The sample was simple in shape and limited in size. The process usually requires expensive Pt electrodes and preheating, and depends strongly on the electrical characteristics of the material. Heterogeneous microstructure. | [52,53] |
| UHS | The electric current generates Joule heating within the carbon felt, which is then transferred to the sample through thermal radiation and conduction. | 103–104 | <1 | Simple equipment, rapid heating rate, and the ability to sinter complex shapes or layered materials. | It cannot be used for sintering materials requiring oxidizing atmosphere, and the temperature control of carbon felt is inadequate. | [43] |
| Sintering Method | Material | Sintering Temperature (°C) | Dwelling Time | Other Sintering Parameters | Average Grain Size (μm) | Density (%) | Conductivity at 850 °C (S/cm) | References |
|---|---|---|---|---|---|---|---|---|
| CS | 8YSZ | 1500 | 12 h | / | 12 | 97.5 | 0.051 | [135] |
| SPS | 8YSZ | 1100 | 480 s | Applied pressure: 110 MPa | 0.21 | 96 | 0.057 | [135] |
| MWS | 8YSZ | 1500 | / | Power: 1.1 kW Frequency: 2.45 GHz | 0.9 | 98 | 0.036 | [136] |
| FS | 8YSZ | 565 (furnace temperature) | 600 s | Electric field: 300 V/cm Current density: 67 mA/mm2 | 0.2 | 96 | 0.056 | [75] |
| UHS | 3YSZ | 1755 | 60 s | Current: 35 A Carbon felt: 80 × 30 × 5 mm3 | 0.18 | 99 | / | [127] |
| 8YSZ | 1439 | 120 s | Current: 13 A Carbon felt: 40 × 9.5 × 4 mm3 | 1.9 | 98.6 | / | [128] |
| Sintering Method | Material | Sintering Temperature (°C) | Dwelling Time | Other Sintering Parameters | Average Grain Size (μm) | Density (%) | Conductivity at 600 °C (S/cm) | References |
|---|---|---|---|---|---|---|---|---|
| CS | 10GDC | 1500 | 10 h | / | 1.2 | 95 | ~0.008 | [137] |
| SPS | 10GDC | 980 | 300 s | Applied pressure: 50 MPa | ~0.2 | 98 | ~0.01 | [138] |
| MWS | 10GDC | 1500 | / | Maximum power: 5 kW Frequency: 2.5 GHz | 0.9 | 95 | ~0.05 | [139] |
| FS | 10GDC | 600 (furnace temperature) | 120 s | Electric field: 100 V/cm Current density: 13.5 mA/mm2 | 0.2 | 98.9 | 0.013 | [65] |
| UHS | 10GDC | >1500 | 360 s | Current: stepwise to 25 A Carbon felt: 30 × 20 × 6 mm3 | / | 95 | / | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wu, X.; Gao, Y.; Yan, Z. Innovations in Electric Current-Assisted Sintering for SOFC: A Review of Advances in Flash Sintering and Ultrafast High-Temperature Sintering. Appl. Sci. 2024, 14, 3953. https://doi.org/10.3390/app14103953
Wu J, Wu X, Gao Y, Yan Z. Innovations in Electric Current-Assisted Sintering for SOFC: A Review of Advances in Flash Sintering and Ultrafast High-Temperature Sintering. Applied Sciences. 2024; 14(10):3953. https://doi.org/10.3390/app14103953
Chicago/Turabian StyleWu, Jiajia, Xiaohu Wu, Yan Gao, and Zilin Yan. 2024. "Innovations in Electric Current-Assisted Sintering for SOFC: A Review of Advances in Flash Sintering and Ultrafast High-Temperature Sintering" Applied Sciences 14, no. 10: 3953. https://doi.org/10.3390/app14103953





