B355252 Suppresses LPS-Induced Neuroinflammation in the Mouse Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animal Cohorts Assignment and Behavior Assessment
2.3. Tissue Sampling
2.4. Histopathology and Hematoxylin/Eosin (H&E) Staining
2.5. Immunohistochemistry (IHC) and Immunofluorescence (IF)
2.6. Nuclear and Cytosolic Fractionation
2.7. Western Blot Analysis
2.8. Cytokine Array
2.9. Statistical Analysis
3. Results
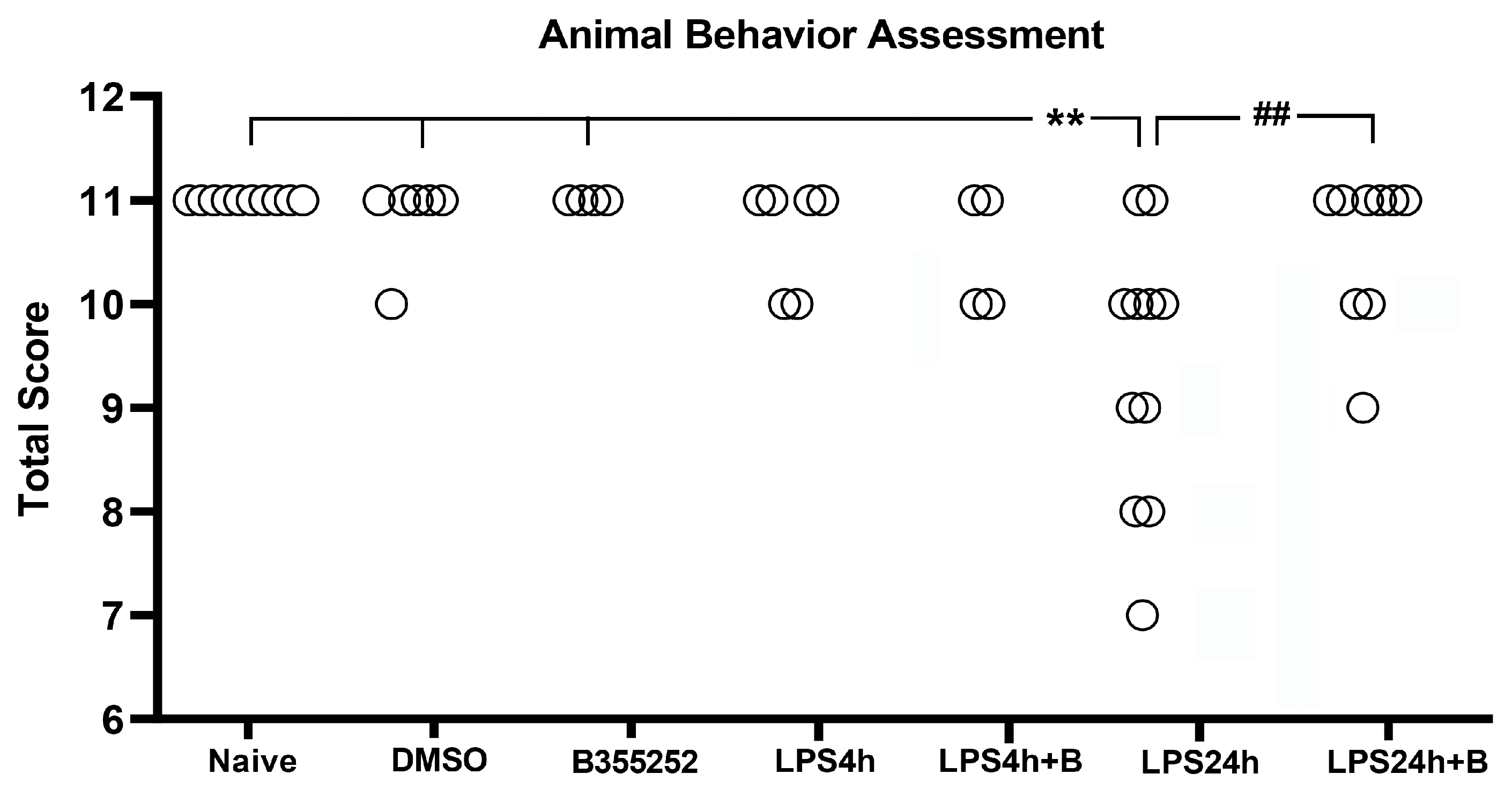
3.1. B355252 Improved Animal Behavior Altered by LPS
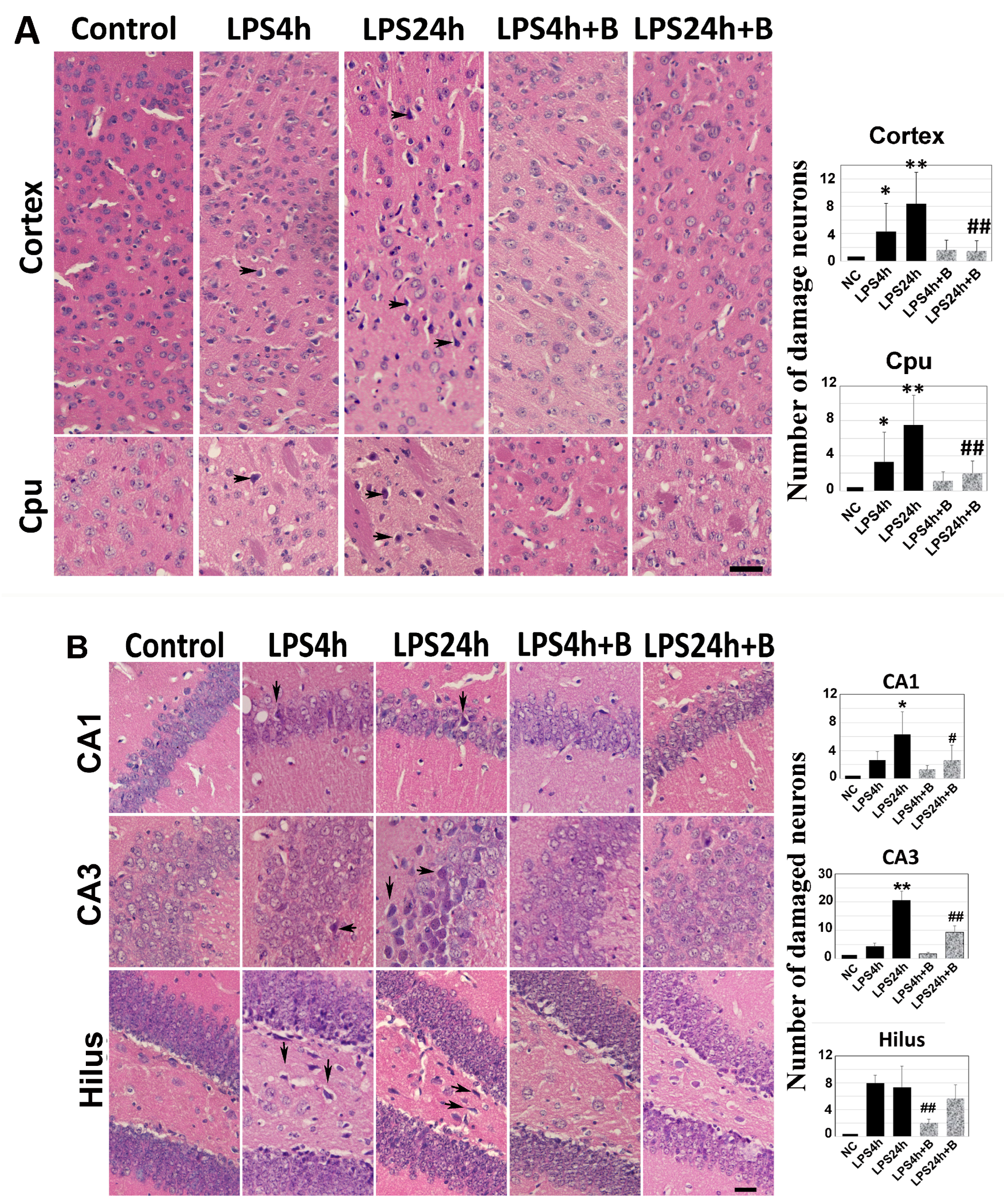
3.2. B355252 Ameliorated LPS-Induced Brain Tissue Damage
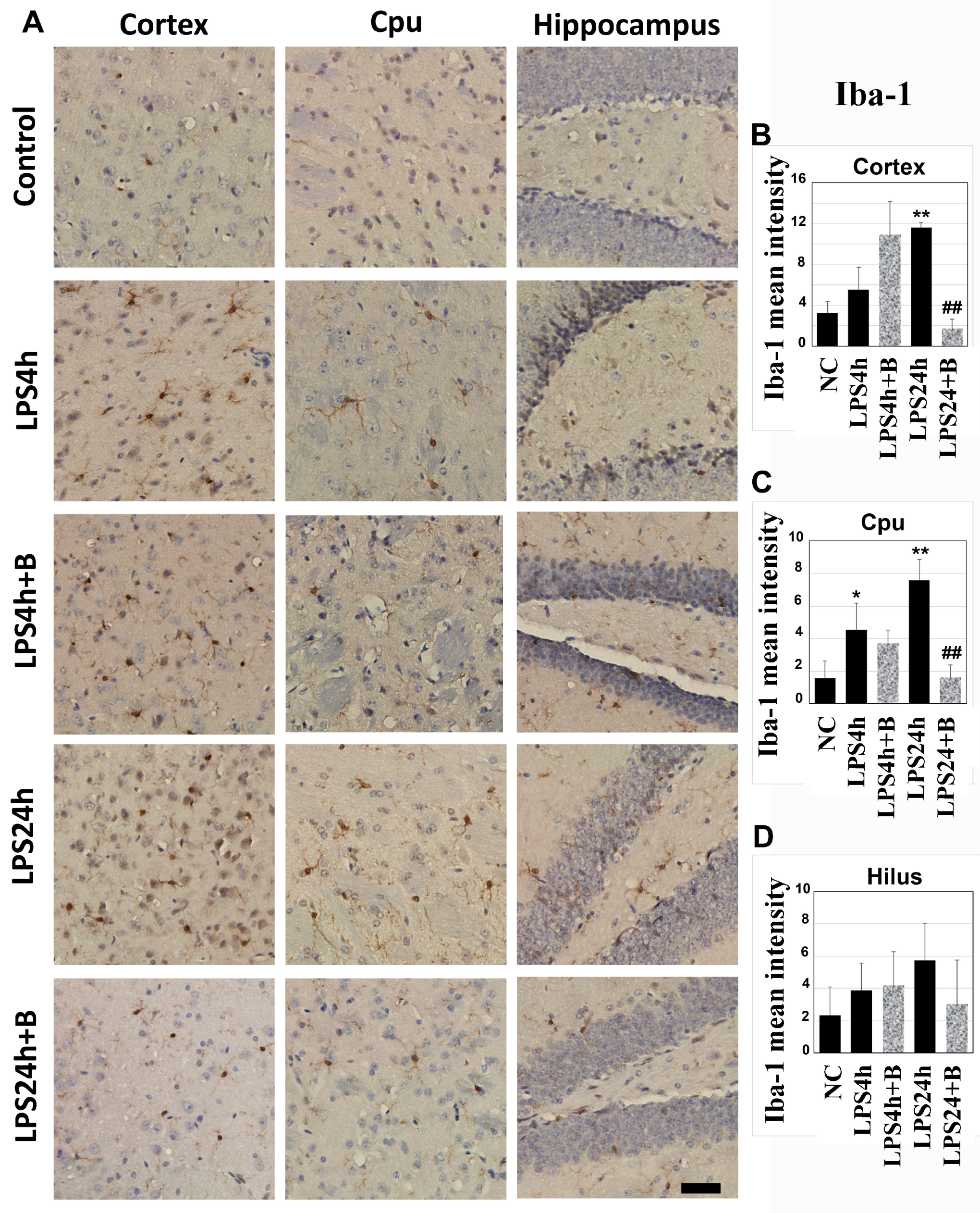
3.3. B355252 Suppressed the Activation of Microglial and Astrocyte Cells Elicited by LPS
3.4. B355252 Reduced Protein Levels of TLR4
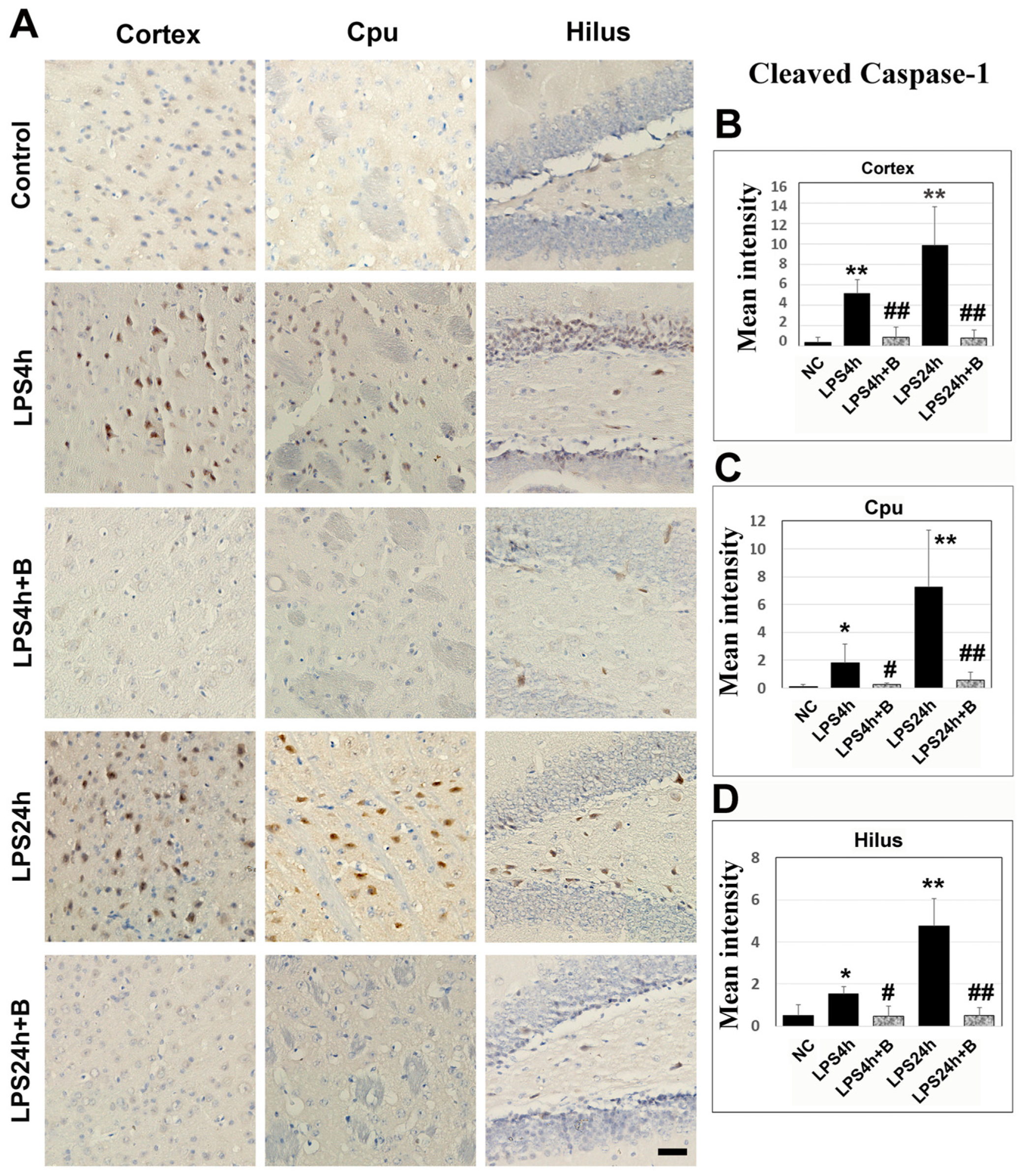
3.5. B355252 Lowered the Levels of NLRP3 and Caspase-1
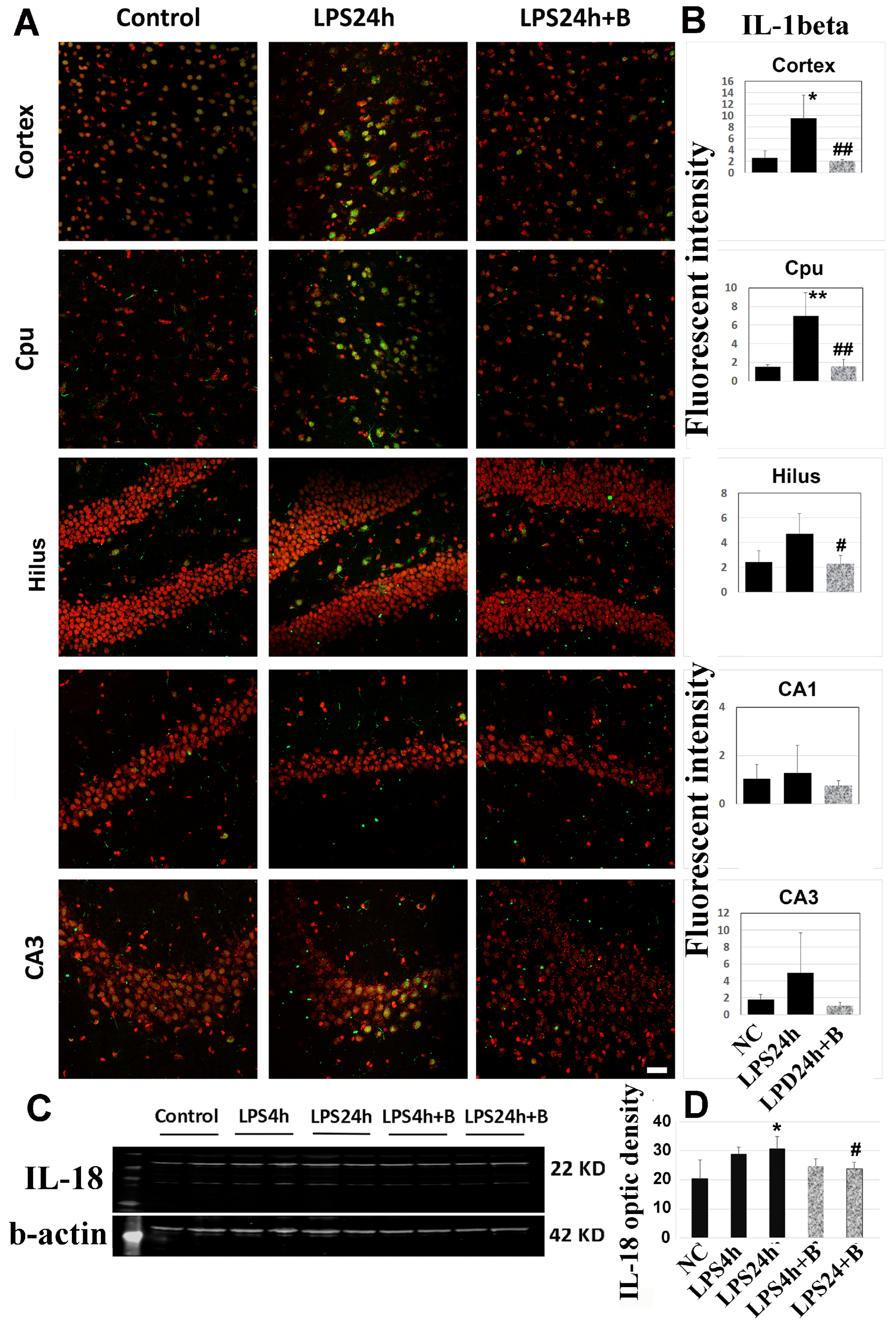
3.6. B355252 Reduced the Levels of IL-1β and IL-18
3.7. B355252 Suppressed the LPS-Elicited Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.L.; Dandepally, S.R.; Gilyazova, N.; Witherspoon, S.M.; Ibeanu, G. Microwave-assisted synthesis of 4-chloro-N-(naphthalen-1-ylmethyl)-5-(3-(piperazin-1-yl)phenoxy)thiophene-2-sulfonamide (B-355252): A new potentiator of Nerve Growth Factor (NGF)-induced neurite outgrowth. Tetrahedron 2010, 66, 9577–9581. [Google Scholar] [CrossRef] [PubMed]
- Yeyeodu, S.T.; Witherspoon, S.M.; Gilyazova, N.; Ibeanu, G.C. A rapid, inexpensive high throughput screen method for neurite outgrowth. Curr. Chem. Genom. 2010, 4, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gliyazova, N.S.; Huh, E.Y.; Ibeanu, G.C. A novel phenoxy thiophene sulphonamide molecule protects against glutamate evoked oxidative injury in a neuronal cell model. BMC Neurosci. 2013, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Gliyazova, N.S.; Ibeanu, G.C. The chemical molecule B355252 is neuroprotective in an in vitro model of Parkinson’s disease. Cell. Mol. Neurobiol. 2016, 36, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Chimeh, U.; Zimmerman, M.A.; Gilyazova, N.; Li, P.A. B355252, A novel small molecule, confers neuroprotection against cobalt chloride toxicity in mouse hippocampal cells through altering mitochondrial dynamics and limiting autophagy induction. Int. J. Med. Sci. 2018, 15, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, Q.; Qi, Q.; Gliyazova, N.S.; Ibeanu, G.; Li, P.A. Neuroprotection by B355252 against glutamate-induced cytotoxicity in murine hippocampal HT-22 cells is associated with activation of ERK3 signaling pathway. Biol. Pharm. Bull. 2021, 44, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gliyazova, N.S.; Li, P.A.; Ibeanu, G. Phenoxythiophene sulfonamide compound B355252 protects neuronal cells against glutamate-induced excitotoxicity by attenuating mitochondrial fission and the nuclear translocation of AIF. Exp. Ther. Med. 2021, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.K.; Chen, J.S.; Hsu, C.Y.; Su, Y.T.; Sung, T.C.; Liang, C.L.; Kwan, A.L.; Wu, C.C. A Novel NGF receptor agonist B355252 ameliorates neuronal loss and inflammatory responses in a rat model of cerebral ischemia. J. Inflamm. Res. 2021, 14, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Gliyazova, N.S.; Dandepally, S.R.; Williams, A.L.; Ibeanu, G.C. Neuroprotective effects of an in vitro BBB permeable phenoxythiophene sulfonamide small molecule in glutamate-induced oxidative injury. Exp. Ther. Med. 2022, 23, 79. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Y.H.; Chen, N.H.; Wang, H.B. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int. Immunopharm. 2019, 67, 458–464. [Google Scholar] [CrossRef]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and Parkinson’s disease—From neurodegeneration to therapeutic opportunities. Cells 2022, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Gevorkian, G. LPS-induced murine neuroinflammation model: Main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, B.; Zhang, S. The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Schronder, K.; Pelegrin, P. NLRP3 and pyroptosis blocker for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Li, C.Y.; Lin, I.L.; Syue, W.J.; Chen, Y.F.; Cheng, K.C.; Teng, Y.N.; Lin, Y.H.; Yen, C.H.; Chiu, C.C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, pyroptosis, and necroptosis-Oh my! The many ways a cell can die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Anderson, M.J.; den Hartigh, A.B.; Fink, S.L. Molecular mechanisms of pyroptosis. In Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2641, pp. 1–16. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Paster, E.V.; Villines, K.A.; Hickman, D.L. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009, 59, 234–241. [Google Scholar] [PubMed]
- Crowe, A.R.; Yue, W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis. Bio Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Update notice: Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis. Bio Protoc. 2023, 13, e4610. [Google Scholar] [CrossRef]
- Gilles, C. Protein Array Analyzer for ImageJ. Bioimage Informatics Index. 2019. Available online: https://biii.eu/protein-array-analyzer-imagej (accessed on 14 February 2024).
- Zhou, H.; Lapointe, B.M.; Clark, S.R.; Zbytnuik, L.; Kubes, P. A Requirement for Microglial TLR4 in Leukocyte Recruitment into Brain in Response to Lipopolysaccharide. J. Immunol. 2006, 177, 177–8103. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-Y.; Gu, X.; Chen, Y.-L.; Liu, J.-B.; Hou, C.-X.; Lin, S.-Y.; Hao, N.-N.; Liang, L.; Chen, W.; Meng, H.-Y. Toll-like receptor 4 activates the NLRP inflammasome pathway and periodontal inflammaging by inhibiting Bmi-1 expression. Int. J. Mol. Med. 2021, 47, 137–150. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K.-I. TLR4 cross-talk with NLRP3 imflammasome and complement signaling pathways in Alzheimer’s disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Nader Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.J.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Z.; He, S.; Zhang, L.; Li, Y. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 11, 768108. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claaen, J.-H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.A. Microglia orchestrate neuroinflammation. eLife 2022, 11, e81890. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Mark, R.E.; Griffin, W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Leyh, J.; Paeschke, S.; Mages, B.; Michalski, D.; Nowicki, M.; Bechmann, I.; Winter, K. Classification of microglial morphological phenotypes using machine learning. Front. Cell. Neurosci. 2021, 15, 701673. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 281–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, F.; Xu, H.; Yang, H.; Shao, M.; Xu, S.; Lyu, F. TLR4 aggravates microglial pyroptosis by promoting NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin. Transl. Med. 2022, 12, e894. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, L.; Wang, L.; Mao, Z.; Zheng, P.; Zhang, F.; Zhang, H.; Liu, H. MUC1 attenuates neutrophilic airway inflammation in asthma by reducing NLRP3 inflammasome-mediated pyroptosis through the inhibition of the TLR4/MyD88/NF-κB pathway. Respir. Res. 2023, 24, 255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-T.; Du, X.-M.; Ma, X.-J.; Zong, Y.; Chen, J.-K.; Yu, C.-L.; Liu, Y.-G.; Chen, Y.-C.; Zhao, L.-J.; Lu, G.-C. Activation of the NLRP3 inflammasome in lipopolysaccharide-indueced mouse fatigue and its relevance to chronic fatigue syndrome. J. Neuroinflamm. 2016, 13, 71. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Si, X.; Liu, H.; Wang, H. The role of pyroptosis and autophagy in ischemia reperfusion Injury. Biomolecules 2022, 12, 1010. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, J.; Song, Y.; Liang, T.; Liu, S.; Ren, C.; Song, X.; Cui, L.; Sun, Y. Pyroptosis and degenerative diseases of the elderly. Cell Death Dis. 2023, 14, 94. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, X.; Huang, L.; Qian, H.; Liu, W. Mechanism of pyroptosis in neurodegenerative diseases and its therapeutic potential by traditional Chinese medicine. Front. Pharmacol. 2023, 14, 1122104. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Wu, M.; Hou, X.; Zhou, Z. What type of cell death occurs in chronic cerebral hypoperfusion? A review focusing on pyroptosis and its potential therapeutic implications. Front. Cell. Neurosci. 2023, 17, 1073511. [Google Scholar] [CrossRef]
- You, H.-M.; Wang, L.; Meng, H.-W.; Huang, C.; Fang, G.-Y.; Li, J. Pyroptosis: Shedding light on the mechanisms and links with cancers. Front. Immunol. 2023, 14, 1290885. [Google Scholar] [CrossRef]
- Pan, Y.; Cai, W.; Huang, J.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. Pyroptosis in development, inflammation and disease. Front. Immunol. 2022, 13, 991044. [Google Scholar] [CrossRef]
- Jin, X.; Ma, Y.; Liu, D.; Huang, Y. Role of pyroptosis in the pathogenesis and treatment of diseases. MedComm 2023, 4, e249. [Google Scholar] [CrossRef]
- Tang, L.; Liu, S.; Li, S.; Chen, Y.; Xie, B.; Zhou, J. Induction mechanism of ferroptosis, necroptosis, and pyroptosis: A novel therapeutic target in nervous system diseases. Int. J. Mol. Sci. 2023, 24, 10127. [Google Scholar] [CrossRef]
- Li, T.; Zheng, G.; Li, B.; Tang, L. Pyroptosis: A promising therapeutic target for noninfectious disease. Cell Prolif. 2021, 54, e13137. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Li, Z.; Mo, F.; Wang, Y.; Li, W.; Chen, Y.; Liu, J.; Chen-Mayfield, T.-J.; Hu, Q. Enhancing Gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nat. Commun. 2022, 13, 6321. [Google Scholar] [CrossRef]
- Niu, Q.; Liu, Y.; Zheng, Y.; Tang, Z.; Qian, Y.; Qi, R.; Shen, J.; Zhao, P. Co-delivery of nigericin and decitabine using hexahistidine-metal nanocarriers for pyroptosis-induced immunotherapeutics. Acta Pharm. Sin. B 2022, 12, 4458–4471. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wan, T.; Gao, X.; Fu, M.; Duan, Y.; Shen, X.; Guo, W. Microglia Pyroptosis: A candidate target for neurological diseases treatment. Front. Endocrinol. 2022, 13, 950798. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Manotovani, S.; Robertson, A.B.A.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, eaah4066. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease—Potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Zhai, Y.; Meng, X.; Ye, T.; Xie, W.; Sun, G.; Sun, X. Inhibiting the NLRP3 Inflammasome Activation with MCC950 Ameliorates Diabetic Encephalopathy in db/db Mice. Molecules 2018, 23, 522. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Li, F.-X.; Gu, R.-N.; Fang, Y.-Y.; Lai, L.-Y.; Wang, Y.-W.; Tao, T.; Xu, S.-Y.; You, Z.-J.; Zhang, H.-F. Inhibition of NLRP3 Inflammasome Ameliorates Cerebral Ischemia-Reperfusion Injury in Diabetic Mice. Neural Plast. 2018, 2018, 9163521. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Zhao, L.; Nasoohi, S.; Ishrat, T. Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci. Rep. 2018, 8, 5971. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Nasoohi, S.; Ishrat, T. MCC950, the Selective Inhibitor of nucleotide oligomerization domain-like receptor protein-3 inflammasome, protects mice against traumatic brain injury. J. Neurotrauma 2018, 35, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhong, W.; Gu, Y.; Li, Y. Emerging mechanisms and targeted therapy of pyroptosis in central nervous system trauma. Front. Cell Dev. Biol. 2022, 10, 832114. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, G. Mechanisms and therapeutic regulation of pyroptosis in inflammatory diseases and cancer. Int. J. Mol. Sci. 2020, 21, 1456. [Google Scholar] [CrossRef]
- Chai, R.; Li, Y.; Shui, L.; Ni, L.; Zhang, A. The role of pyroptosis in inflammatory diseases. Front. Cell Dev. Biol. 2023, 11, 1173235. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rong, R.; Xia, X. Spotlight on pyroptosis: Role in pathogenesis and therapeutic potential of ocular diseases. J. Neuroinflamm. 2022, 19, 183. [Google Scholar] [CrossRef] [PubMed]
- Phulphagar, K.; Kuhn, L.; Ebner, S.; Frauenstein, A.; Swietlik, J.J.; Rieckmann, J.; Meissner, F. Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis. Cell Rep. 2021, 34, 108826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Chen, Q.; Jiao, F.; Shi, C.; Pei, M.; Lv, J.; Zhang, H.; Wang, L.; Gong, Z. TNF-alpha/HMGB1 inflammation signaling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020, 53, e12829. [Google Scholar] [CrossRef]
- Ploix, C.C.; Noor, S.; Crane, J.; Masek, K.; Carter, W.; Lo, D.D.; Wilson, E.H.; Carson, J. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav. Immun. 2011, 25, 883–896. [Google Scholar] [CrossRef]
- Chen, S.C.; Leach, M.W.; Chen, Y.; Cai, X.Y.; Sullivan, L.; Wiekowski, M.; Dovey-Hartman, B.J.; Zlotnik, A.; Lira, S.A. Central nervous system inflammation and neurological disease in transgenic mice expressing the cc chemokine CCL21 in oligodendrocytes. J. Immunol. 2002, 168, 1009–1017. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.J.; Kim, J.M.; Kang, H.J.; Cho, S.H.; Chang, S.E. Epidermal growth factor relieves inflammatory signals in Staphylococcus aureus-treated human epidermal keratinocytes and atopic dermatitis-like skin lesions in Nc/Nga mice. BioMed Res. Int. 2018, 2018, 9439182. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.H.; Cantrell, D.A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Zhang, P.; Catterson, J.H.; Gronke, S.; Patridge, L. Inhibition of S6K lowers age-related inflammation and increases lifespan through the endolysosomal system. Nat. Aging 2024, 4, 491–509. [Google Scholar] [CrossRef]
- Hannocks, M.-J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Qi, Q.; Ibeanu, G.C.; Li, P.A. B355252 Suppresses LPS-Induced Neuroinflammation in the Mouse Brain. Brain Sci. 2024, 14, 467. https://doi.org/10.3390/brainsci14050467
He Q, Qi Q, Ibeanu GC, Li PA. B355252 Suppresses LPS-Induced Neuroinflammation in the Mouse Brain. Brain Sciences. 2024; 14(5):467. https://doi.org/10.3390/brainsci14050467
Chicago/Turabian StyleHe, Qingping, Qi Qi, Gordon C. Ibeanu, and P. Andy Li. 2024. "B355252 Suppresses LPS-Induced Neuroinflammation in the Mouse Brain" Brain Sciences 14, no. 5: 467. https://doi.org/10.3390/brainsci14050467





