Cholinergic Interneurons in the Accumbal Shell Region Regulate Binge Alcohol Self-Administration in Mice: An In Vivo Calcium Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Surgeries for Intra-NAcSh Delivery of Calcium Indicator (GCaMP)
2.3. Habituation
2.4. Baseline Recording
2.5. Binge Drinking
2.6. Calcium Imaging Procedure
2.7. Processing and Analysis of Calcium Imaging Videos
2.8. Data Analysis and Visualization
2.9. Blood Alcohol Concentration
2.10. Immunofluorescence
2.11. Microscopy and Quantification
2.12. Statistical Design and Analysis
3. Results
Localization of Lens Implantation Site and GCAMP6s Expression in CIN Neurons
- (a)
- Alcohol consumption: In the alcohol group, mice displayed a consistent amount of alcohol consumption, with 3.4 ± 0.1 g/kg on day 1, 3.7 ± 0.2 g/kg on day 2, and 4.0 ± 0.1 g/kg on day 3, with no significant difference (F2,9 = 1.50; p = 0.27). On day 4, the mean ± SEM amount of alcohol consumption was 5.3 ± 0.1 g/kg, with a BAC of 102.6 ± 1.6 g/dL, suggesting binge alcohol consumption.
- (b)
- Sucrose consumption: In the sucrose group, mice displayed a consistent amount of sucrose consumption, with 9.64 ± 0.031 g/kg on day 1, 9.95 ± 0.29 g/kg on day 2, and 10.11 ± 0.09 g/kg on day 3, with no significant difference (F2,9 = 0.94; p = 0.43). On day 4, the mean ± SEM amount of sucrose consumption was 13.88 ± 0.52 g/kg.
- (c)
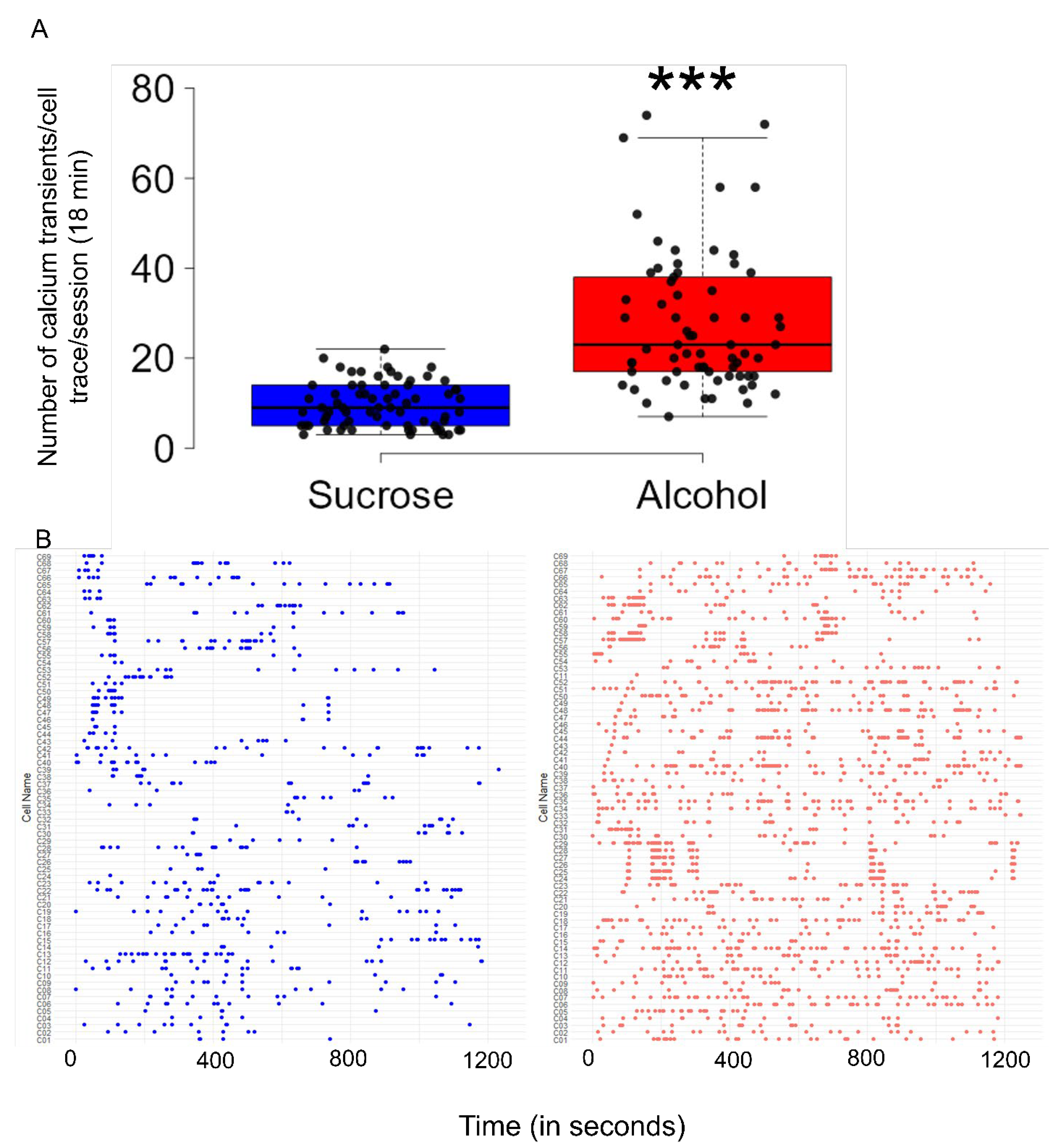
- Binge alcohol consumption increases the CIN activity in the NAcSh.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apicella, P. The role of the intrinsic cholinergic system of the striatum: What have we learned from TAN recordings in behaving animals? Neuroscience 2017, 360, 81–94. [Google Scholar] [CrossRef]
- Berlanga, M.L.; Olsen, C.M.; Chen, V.; Ikegami, A.; Herring, B.E.; Duvauchelle, C.L.; Alcantara, A.A. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience 2003, 120, 1149–1156. [Google Scholar] [CrossRef]
- Witten, I.B.; Lin, S.C.; Brodsky, M.; Prakash, R.; Diester, I.; Anikeeva, P.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 2010, 330, 1677–1681. [Google Scholar] [CrossRef]
- Yorgason, J.T.; Zeppenfeld, D.M.; Williams, J.T. Cholinergic Interneurons Underlie Spontaneous Dopamine Release in Nucleus Accumbens. J. Neurosci. 2017, 37, 2086–2096. [Google Scholar] [CrossRef]
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64. [Google Scholar] [CrossRef]
- Cachope, R.; Mateo, Y.; Mathur, B.N.; Irving, J.; Wang, H.L.; Morales, M.; Lovinger, D.M.; Cheer, J.F. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Rep. 2012, 2, 33–41. [Google Scholar] [CrossRef]
- Hanada, Y.; Kawahara, Y.; Ohnishi, Y.N.; Shuto, T.; Kuroiwa, M.; Sotogaku, N.; Greengard, P.; Sagi, Y.; Nishi, A. p11 in Cholinergic Interneurons of the Nucleus Accumbens Is Essential for Dopamine Responses to Rewarding Stimuli. eNeuro 2018, 5, e0332-18. [Google Scholar] [CrossRef]
- Gonzales, K.K.; Smith, Y. Cholinergic interneurons in the dorsal and ventral striatum: Anatomical and functional considerations in normal and diseased conditions. Ann. N. Y. Acad. Sci. 2015, 1349, 1–45. [Google Scholar] [CrossRef]
- Abudukeyoumu, N.; Hernandez-Flores, T.; Garcia-Munoz, M.; Arbuthnott, G.W. Cholinergic modulation of striatal microcircuits. Eur. J. Neurosci. 2019, 49, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Sharma, R.; Mishra, V.; Sahota, P.; Thakkar, M. Genetic ablation of cholinergic interneurons in the medial shell region of the nucleus accumbens reduces binge drinking in C57BL/6J mice. Soc. Neurosci. Abstr. Neurosci. Meet. Plan. Virtual Soc. Neurosci. 2021, 2021. Available online: https://www.abstractsonline.com/pp8/?_gl=1*gxybh2*_ga*NDIyMjYyMjk3LjE3MTUwMTg1Mjk.*_ga_T09K3Q2WDN*MTcxNTI3NjcyNS40LjAuMTcxNTI3NjcyNS42MC4wLjA.#!/10485/participants/Parikh/1 (accessed on 3 April 2024).
- Sharma, R.; Chischolm, A.; Parikh, M.; Thakkar, M. Cholinergic interneurons in the shell region of the nucleus accumbens regulate binge alcohol consumption: A chemogenetic and genetic lesion study. Alcohol Clin. Exp. Res. 2024, 48, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Herring, B.E.; Mayfield, R.D.; Camp, M.C.; Alcantara, A.A. Ethanol-Induced Fos Immunoreactivity in the Extended Amygdala and Hypothalamus of the Rat Brain: Focus on Cholinergic Interneurons of the Nucleus Accumbens. Alcohol Clin. Exp. Res. 2006, 28, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Camp, M.C.; Mayfield, R.D.; McCracken, M.; McCracken, L.; Alcantara, A.A. Neuroadaptations of Cdk5 in Cholinergic Interneurons of the Nucleus Accumbens and Prefrontal Cortex of Inbred Alcohol-preferring Rats Following Voluntary Alcohol Drinking. Alcohol Clin. Exp. Res. 2006, 30, 1322–1335. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Best, K.; Belknap, J.K.; Finn, D.A.; Crabbe, J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.S.; Ford, M.M.; Yu, C.H.; Brown, L.L.; Finn, D.A.; Garland, T., Jr.; Crabbe, J.C. Mouse inbred strain differences in ethanol drinking to intoxication. Genes. Brain Behav. 2007, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sprow, G.M.; Thiele, T.E. The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol. Behav. 2012, 106, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.E.; Navarro, M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol 2014, 48, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Zhou, P.; Resendez, S.L.; Rodriguez-Romaguera, J.; Jimenez, J.C.; Neufeld, S.Q.; Giovannucci, A.; Friedrich, J.; Pnevmatikakis, E.A.; Stuber, G.D.; Hen, R. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife 2018, 7, e28728. [Google Scholar] [CrossRef]
- Berdyyeva, T.K.; Frady, E.P.; Nassi, J.J.; Aluisio, L.; Cherkas, Y.; Otte, S.; Wyatt, R.M.; Dugovic, C.; Ghosh, K.K.; Schnitzer, M.J.; et al. Direct Imaging of Hippocampal Epileptiform Calcium Motifs Following Kainic Acid Administration in Freely Behaving Mice. Front. Neurosci. 2016, 10, 53. [Google Scholar] [CrossRef]
- Mukamel, E.A.; Nimmerjahn, A.; Schnitzer, M.J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 2009, 63, 747–760. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Rapid tolerance development to the NREM sleep promoting effect of alcohol. Sleep 2014, 37, 821–824. [Google Scholar] [CrossRef]
- Sharma, R.; Engemann, S.; Sahota, P.; Thakkar, M.M. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J. Neurochem. 2010, 115, 782–794. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Role of adenosine and the orexinergic perifornical hypothalamus in sleep-promoting effects of ethanol. Sleep 2014, 37, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Troger, J.; Hoischen, C.; Perner, B.; Monajembashi, S.; Barbotin, A.; Loschberger, A.; Eggeling, C.; Kessels, M.M.; Qualmann, B.; Hemmerich, P. Comparison of Multiscale Imaging Methods for Brain Research. Cells 2020, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- R-CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Crabbe, J.C.; Harris, R.A.; Koob, G.F. Preclinical studies of alcohol binge drinking. Ann. N. Y. Acad. Sci. 2011, 1216, 24–40. [Google Scholar] [CrossRef] [PubMed]
- NIAAA. Alcohol-Related Emergencies and Deaths in the United States. 2023. Available online: https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states#:~:text=The%20Alcohol%2DRelated%20Disease%20Impact,behind%20tobacco%2C%20poor%20diet%20and (accessed on 3 April 2024).
- Di Chiara, G. Alcohol and dopamine. Alcohol Health Res. World 1997, 21, 108–114. [Google Scholar]
- Clarke, R.; Adermark, L. Dopaminergic Regulation of Striatal Interneurons in Reward and Addiction: Focus on Alcohol. Neural Plast. 2015, 2015, 814567. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.B.; Adermark, L.; Chau, P.; Soderpalm, B.; Ericson, M. Increase in nucleus accumbens dopamine levels following local ethanol administration is not mediated by acetaldehyde. Alcohol Alcohol. 2014, 49, 498–504. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Soyka, M. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 2018, 320, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Stahre, M.; Roeber, J.; Kanny, D.; Brewer, R.D.; Zhang, X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev. Chronic. Dis. 2014, 11, e109. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Parikh, M.; Mishra, V.; Soni, A.; Rubi, S.; Sahota, P.; Thakkar, M. Antisense-induced downregulation of major circadian genes modulates the expression of histone deacetylase-2 (HDAC-2) and CREB-binding protein (CBP) in the medial shell region of nucleus accumbens of mice exposed to chronic excessive alcohol consumption. J. Neurochem. 2022, 161, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Puckett, H.; Kemerling, M.; Parikh, M.; Sahota, P.; Thakkar, M. Antisense-Induced Downregulation of Clock Genes in the Shell Region of the Nucleus Accumbens Reduces Binge Drinking in Mice. Alcohol Clin. Exp. Res. 2021, 45, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mishra, V.; Parikh, M.; Soni, A.; Sahota, P.; Thakkar, M. Antisense-induced knockdown of cAMP response element-binding protein downregulates Per1 gene expression in the shell region of nucleus accumbens resulting in reduced alcohol consumption in mice. Alcohol Clin. Exp. Res. 2021, 45, 1940–1949. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Nicotine Administration in the Cholinergic Basal Forebrain Increases Alcohol Consumption in C57BL/6J Mice. Alcohol Clin. Exp. Res. 2014, 38, 1315–1320. [Google Scholar] [CrossRef]
- Lee, J.H.; Ribeiro, E.A.; Kim, J.; Ko, B.; Kronman, H.; Jeong, Y.H.; Kim, J.K.; Janak, P.H.; Nestler, E.J.; Koo, J.W.; et al. Dopaminergic Regulation of Nucleus Accumbens Cholinergic Interneurons Demarcates Susceptibility to Cocaine Addiction. Biol. Psychiatry 2020, 88, 746–757. [Google Scholar] [CrossRef]
- Mark, G.P.; Shabani, S.; Dobbs, L.K.; Hansen, S.T. Cholinergic modulation of mesolimbic dopamine function and reward. Physiol. Behav. 2011, 104, 76–81. [Google Scholar] [CrossRef]
- Kolpakova, J.; van der Vinne, V.; Gimenez-Gomez, P.; Le, T.; Martin, G.E. Binge alcohol drinking alters the differential control of cholinergic interneurons over nucleus accumbens D1 and D2 medium spiny neurons. Front. Cell Neurosci. 2022, 16, 1010121. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.M.; Schachter, M.J.; Gulati, S.; Zitelli, K.T.; Malanowski, S.; Tajik, A.; Fritz, C.; Trulson, M.; Otte, S.L. Simultaneous Optogenetics and Cellular Resolution Calcium Imaging During Active Behavior Using a Miniaturized Microscope. Front. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Ziv, Y.; Ghosh, K.K. Miniature microscopes for large-scale imaging of neuronal activity in freely behaving rodents. Curr. Opin. Neurobiol. 2015, 32, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.H.; Ung, R.L.; Resendez, S.L.; Stamatakis, A.M.; Taylor, J.G.; Huang, J.; Veleta, K.; Kantak, P.A.; Aita, M.; Shilling-Scrivo, K.; et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 2015, 160, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Shiromani, P.J.; Blanco-Centurion, C.; Vidal-Ortiz, A. Mapping Network Activity in Sleep. Front. Neurosci. 2021, 15, 646468. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Centurion, C.; Luo, S.; Spergel, D.J.; Vidal-Ortiz, A.; Oprisan, S.A.; Van den Pol, A.N.; Liu, M.; Shiromani, P.J. Dynamic Network Activation of Hypothalamic MCH Neurons in REM Sleep and Exploratory Behavior. J. Neurosci. 2019, 39, 4986–4998. [Google Scholar] [CrossRef] [PubMed]
- Kostin, A.; Alam, M.A.; Saevskiy, A.; Yang, C.; Golshani, P.; Alam, M.N. Calcium Dynamics of the Ventrolateral Preoptic GABAergic Neurons during Spontaneous Sleep-Waking and in Response to Homeostatic Sleep Demands. Int. J. Mol. Sci. 2023, 24, 8311. [Google Scholar] [CrossRef] [PubMed]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef]
- Di Chiara, G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Rivera-Irizarry, J.K.; Zallar, L.J.; Levine, O.B.; Skelly, M.J.; Boyce, J.E.; Barney, T.; Kopyto, R.; Pleil, K.E. Sex differences in binge alcohol drinking and the behavioral consequences of protracted abstinence in C57BL/6J mice. Biol. Sex Differ. 2023, 14, 83. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Chischolm, A.; Parikh, M.; Kempuraj, D.; Thakkar, M. Cholinergic Interneurons in the Accumbal Shell Region Regulate Binge Alcohol Self-Administration in Mice: An In Vivo Calcium Imaging Study. Brain Sci. 2024, 14, 484. https://doi.org/10.3390/brainsci14050484
Sharma R, Chischolm A, Parikh M, Kempuraj D, Thakkar M. Cholinergic Interneurons in the Accumbal Shell Region Regulate Binge Alcohol Self-Administration in Mice: An In Vivo Calcium Imaging Study. Brain Sciences. 2024; 14(5):484. https://doi.org/10.3390/brainsci14050484
Chicago/Turabian StyleSharma, Rishi, Abigail Chischolm, Meet Parikh, Deepak Kempuraj, and Mahesh Thakkar. 2024. "Cholinergic Interneurons in the Accumbal Shell Region Regulate Binge Alcohol Self-Administration in Mice: An In Vivo Calcium Imaging Study" Brain Sciences 14, no. 5: 484. https://doi.org/10.3390/brainsci14050484




