Neural Adaptation at Stimulus Onset and Speed of Neural Processing as Critical Contributors to Speech Comprehension Independent of Hearing Threshold or Age
Abstract
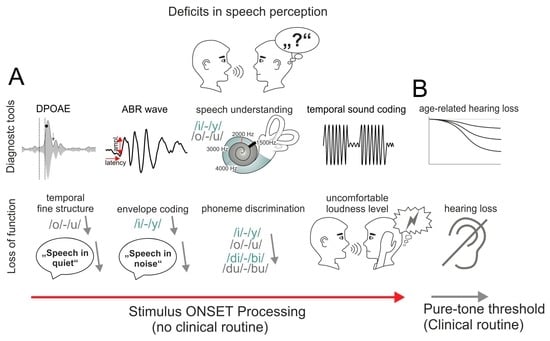
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychiatric Scores
2.3. Otoscopy and Impedance Audiometry
2.4. Pure-Tone Audiometry
2.5. Auditory Brainstem Responses (ABRs)
2.6. Auditory Steady-State Response (ASSR)
2.7. Distortion-Product Otoacoustic Emissions (DPOAEs)
2.8. Speech Reception Thresholds (OLSA)
2.9. Pure-Tone-Normalized OLSA Threshold
2.10. Stimuli for Phoneme Discrimination
2.11. Behavioral Phoneme Discrimination Task
2.12. Statistical Analysis
2.13. Pure-Tone-Normalized OLSA (PNOT)
2.14. Variance Analysis
2.15. Data Distributions
3. Results
3.1. Pure-Tone Thresholds Are Elevated with Age
3.2. Speech Reception Thresholds Elevate with PTA-Threshold and Age
3.3. The Supra-Threshold ABR Wave Decreases with Elevated, Age-Dependent PTA-EHF
3.4. Speech Comprehension Exhibits Components That Are Dependent on and Independent of Pure-Tone Threshold and Age
3.5. Differences between Good and Poor Pure-Tone-Normalized OLSA Thresholds (PNOTs) Is a Better Indicator of Self-Assessed Hearing Ability than Age
3.6. The Difference between Good and Poor PNOTs Shows Low Dependence on Temporal Envelope (TENV) Coding (ASSR)
3.7. The Difference between Good and Poor PNOT Is Reflected in Differences in Cochlear Amplifier Efficiency at Stimulus Onset
3.8. The Difference between Good and Poor PNOTs Is Reflected in Variations in Supra-Threshold Amplitude and Response Latencies of ANFs
3.9. Delta to Poor and Good PNOTs Show Differences in Phoneme Discrimination below and above the PLL
4. Discussion
4.1. PTTs and SRT50 Show Age-Dependent Differences
4.2. Supra-Threshold ABR Wave Decrease with Elevated Age-Dependent PTA-EHF
4.3. Difference between Good and Poor PNOTs Is a Better Indicator of Self-Assessed Hearing Ability than Age
4.4. Difference between Good and Poor PNOTs Show Low Dependence on Temporal Coding (ASSR)
4.5. Differences between Good and Poor PNOTs Are Reflected in Variations in Cochlear Amplifier Efficacy at Stimulus Onset
4.6. Differences in Good and Poor PNOTs Are Reflected in Variations in Supra-Threshold Amplitude and Response Latencies of Auditory Nerve Fibers
4.7. Differences in Good and Poor PNOTs Are Reflected in Variations in Phoneme Discrimination below and above the PLL
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Golub, J.S.; Brickman, A.M.; Ciarleglio, A.J.; Schupf, N.; Luchsinger, J.A. Association of Subclinical Hearing Loss With Cognitive Performance. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.; Hocke, T.; Iro, H. Age-Related Decline of Speech Perception. Front. Aging Neurosci. 2022, 14, 891202. [Google Scholar] [CrossRef] [PubMed]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef] [PubMed]
- Mohrle, D.; Ni, K.; Varakina, K.; Bing, D.; Lee, S.C.; Zimmermann, U.; Knipper, M.; Ruttiger, L. Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiol. Aging 2016, 44, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.J.M.; Garcia-Lazaro, J.A.; McAlpine, D.; Schaette, R. Hidden Hearing Loss Impacts the Neural Representation of Speech in Background Noise. Curr. Biol. 2020, 30, 4710–4721.e4. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, H.M.; Mai, A.R.; Simpson, J.M.; Choi, I.; Heinz, M.G.; Shinn-Cunningham, B.G. Non-Invasive Assays of Cochlear Synaptopathy—Candidates and Considerations. Neuroscience 2019, 407, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Mepani, A.M.; Verhulst, S.; Hancock, K.E.; Garrett, M.; Vasilkov, V.; Bennett, K.; de Gruttola, V.; Liberman, M.C.; Maison, S.F. Envelope following responses predict speech-in-noise performance in normal-hearing listeners. J. Neurophysiol. 2021, 125, 1213–1222. [Google Scholar] [CrossRef]
- Viana, L.M.; O’Malley, J.T.; Burgess, B.J.; Jones, D.D.; Oliveira, C.A.; Santos, F.; Merchant, S.N.; Liberman, L.D.; Liberman, M.C. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 2015, 327, 78–88. [Google Scholar] [CrossRef]
- Kobel, M.; Le Prell, C.G.; Liu, J.; Hawks, J.W.; Bao, J. Noise-induced cochlear synaptopathy: Past findings and future studies. Hear. Res. 2017, 349, 148–154. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Plack, C.J.; Barker, D.; Prendergast, G. Perceptual consequences of “hidden” hearing loss. Trends Hear. 2014, 18, 2331216514550621. [Google Scholar] [CrossRef] [PubMed]
- Fullgrabe, C.; Moore, B.C.; Stone, M.A. Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Front. Aging Neurosci. 2014, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Frisina, R.D. Age-related hearing loss: Ear and brain mechanisms. Ann. N. Y. Acad. Sci. 2009, 1170, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Z.; Liberman, L.D.; Bennett, K.; de Gruttola, V.; O’Malley, J.T.; Liberman, M.C. Primary Neural Degeneration in the Human Cochlea: Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience 2019, 407, 8–20. [Google Scholar] [CrossRef]
- Chambers, A.R.; Resnik, J.; Yuan, Y.; Whitton, J.P.; Edge, A.S.; Liberman, M.C.; Polley, D.B. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 2016, 89, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Bakay, W.M.H.; Anderson, L.A.; Garcia-Lazaro, J.A.; McAlpine, D.; Schaette, R. Hidden hearing loss selectively impairs neural adaptation to loud sound environments. Nat. Commun. 2018, 9, 4298. [Google Scholar] [CrossRef]
- Asokan, M.M.; Williamson, R.S.; Hancock, K.E.; Polley, D.B. Publisher Correction: Sensory overamplification in layer 5 auditory corticofugal projection neurons following cochlear nerve synaptic damage. Nat. Commun. 2018, 9, 3158. [Google Scholar] [CrossRef]
- Hesse, L.L.; Bakay, W.; Ong, H.C.; Anderson, L.; Ashmore, J.; McAlpine, D.; Linden, J.; Schaette, R. Non-Monotonic Relation between Noise Exposure Severity and Neuronal Hyperactivity in the Auditory Midbrain. Front. Neurol. 2016, 7, 133. [Google Scholar] [CrossRef]
- Kraus, N.; Anderson, S.; White-Schwoch, T. The Frequency-Following Response: A Window into Human Communication. In The Frequency-Following Response: A Window into Human Communication; Kraus, N., Anderson, S., White-Schwoch, T., Fay, R.R., Popper, A.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–15. [Google Scholar]
- Chen, G.D. Hidden cochlear impairments. J. Otol. 2018, 13, 37–43. [Google Scholar] [CrossRef]
- De Siati, R.D.; Rosenzweig, F.; Gersdorff, G.; Gregoire, A.; Rombaux, P.; Deggouj, N. Auditory Neuropathy Spectrum Disorders: From Diagnosis to Treatment: Literature Review and Case Reports. J. Clin. Med. 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Iliadou, V.V.; Ptok, M.; Grech, H.; Pedersen, E.R.; Brechmann, A.; Deggouj, N.; Kiese-Himmel, C.; Sliwinska-Kowalska, M.; Nickisch, A.; Demanez, L.; et al. A European Perspective on Auditory Processing Disorder-Current Knowledge and Future Research Focus. Front. Neurol. 2017, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Yingling, C.D.; Gardi, J.N. Intraoperative monitoring of facial and cochlear nerves during acoustic neuroma surgery. Otolaryngol. Clin. North Am. 1992, 25, 413–448. [Google Scholar] [CrossRef] [PubMed]
- Eggink, M.C.; Frijns, J.H.M.; Sagers, J.E.; O’Malley, J.T.; Liberman, M.C.; Stankovic, K.M. Human vestibular schwannoma reduces density of auditory nerve fibers in the osseous spiral lamina. Hear. Res. 2022, 418, 108458. [Google Scholar] [CrossRef] [PubMed]
- Le Prell, C.G. Effects of noise exposure on auditory brainstem response and speech-in-noise tasks: A review of the literature. Int J. Audiol. 2019, 58, S3–S32. [Google Scholar] [CrossRef] [PubMed]
- Snell, K.B.; Frisina, D.R. Relationships among age-related differences in gap detection and word recognition. J. Acoust. Soc. Am. 2000, 107, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, P.T.; Perez-Gonzalez, P.; Kalluri, S.; Blanco, J.L.; Lopez-Poveda, E.A. The Influence of Cochlear Mechanical Dysfunction, Temporal Processing Deficits, and Age on the Intelligibility of Audible Speech in Noise for Hearing-Impaired Listeners. Trends Hear. 2016, 20, 2331216516641055. [Google Scholar] [CrossRef]
- Marrufo-Perez, M.I.; Lopez-Poveda, E.A. Adaptation to noise in normal and impaired hearing. J. Acoust. Soc. Am. 2022, 151, 1741. [Google Scholar] [CrossRef]
- Ayasse, N.D.; Penn, L.R.; Wingfield, A. Variations Within Normal Hearing Acuity and Speech Comprehension: An Exploratory Study. Am. J. Audiol. 2019, 28, 369–375. [Google Scholar] [CrossRef]
- Smith, R.L.; Zwislocki, J.J. Short-term adaptation and incremental responses of single auditory-nerve fibers. Biol. Cybern. 1975, 17, 169–182. [Google Scholar] [CrossRef]
- von Gablenz, P.; Holube, I. Hearing Loss and Speech Recognition in the Elderly. Laryngorhinootologie 2017, 96, 759–764. [Google Scholar] [CrossRef] [PubMed]
- den Besten, C.A.; Monksfield, P.; Bosman, A.; Skarzynski, P.H.; Green, K.; Runge, C.; Wigren, S.; Blechert, J.I.; Flynn, M.C.; Mylanus, E.A.M.; et al. Audiological and clinical outcomes of a transcutaneous bone conduction hearing implant: Six-month results from a multicentre study. Clin. Otolaryngol. 2019, 44, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Monson, B.B.; Hunter, E.J.; Lotto, A.J.; Story, B.H. The perceptual significance of high-frequency energy in the human voice. Front. Psychol. 2014, 5, 587. [Google Scholar] [CrossRef] [PubMed]
- Sliwinska-Kowalska, M. Hearing. Handb. Clin. Neurol. 2015, 131, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L.L.; Monson, B.B.; Moore, D.R.; Dhar, S.; Wright, B.A.; Munro, K.J.; Zadeh, L.M.; Blankenship, C.M.; Stiepan, S.M.; Siegel, J.H. Extended high frequency hearing and speech perception implications in adults and children. Hear. Res. 2020, 397, 107922. [Google Scholar] [CrossRef]
- Lorenzi, C.; Gilbert, G.; Carn, H.; Garnier, S.; Moore, B.C. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. Proc. Natl. Acad. Sci. USA 2006, 103, 18866–18869. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, L.A.; Valero, M.D.; Liberman, M.C. Towards a Diagnosis of Cochlear Neuropathy with Envelope Following Responses. J. Assoc. Res. Otolaryngol. 2015, 16, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Bartlett, E.L.; Kujawa, S.G. Age-related Changes in Neural Coding of Envelope Cues: Peripheral Declines and Central Compensation. Neuroscience 2019, 407, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.; Vasilkov, V.; Mauermann, M.; Wilson, J.L.; Henry, K.S.; Verhulst, S. Speech-in-noise intelligibility difficulties with age: The role of cochlear synaptopathy. bioRxiv 2024, 2020.06.09.142950. [Google Scholar] [CrossRef]
- Rance, G. The Auditory Steady-State Response: Generation, Recording, and Clinical Application; Plural Publishing: San Diego, CA, USA, 2008. [Google Scholar]
- Kuwada, S.; Anderson, J.S.; Batra, R.; Fitzpatrick, D.C.; Teissier, N.; D’Angelo, W.R. Sources of the scalp-recorded amplitude-modulation following response. J. Am. Acad. Audiol. 2002, 13, 188–204. [Google Scholar] [CrossRef]
- Lu, H.; Mehta, A.H.; Oxenham, A.J. Methodological considerations when measuring and analyzing auditory steady-state responses with multi-channel EEG. Curr. Res. Neurobiol. 2022, 3, 100061. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.; Lorenz, L.; Thiericke, J.P.; Gummer, A.W.; Dalhoff, E. Input-output functions of the nonlinear-distortion component of distortion-product otoacoustic emissions in normal and hearing-impaired human ears. J. Acoust. Soc. Am. 2017, 141, 3203. [Google Scholar] [CrossRef]
- Portmann, M.; Cazals, Y.; Negrevergne, M.; Aran, J.M. Transtympanic and surface recordings in the diagnosis of retrocochlear disorders. Acta Oto-Laryngol. 1980, 89, 362–369. [Google Scholar] [CrossRef]
- Wei, L.; Ding, D.; Sun, W.; Xu-Friedman, M.A.; Salvi, R. Effects of sodium salicylate on spontaneous and evoked spike rate in the dorsal cochlear nucleus. Hear. Res. 2010, 267, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Melcher, J.R.; Guinan, J.J., Jr.; Knudson, I.M.; Kiang, N.Y. Generators of the brainstem auditory evoked potential in cat. II. Correlating lesion sites with waveform changes. Hear. Res. 1996, 93, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jannetta, P.J.; Jho, H.D. Click-evoked responses from the cochlear nucleus: A study in human. Electroencephalogr. Clin. Neurophysiol. 1994, 92, 215–224. [Google Scholar] [CrossRef]
- Hashimoto, I.; Ishiyama, Y.; Yoshimoto, T.; Nemoto, S. Brain-stem auditory-evoked potentials recorded directly from human brain-stem and thalamus. Brain 1981, 104, 841–859. [Google Scholar] [CrossRef]
- Hofmeier, B.; Wolpert, S.; Aldamer, E.S.; Walter, M.; Thiericke, J.; Braun, C.; Zelle, D.; Ruttiger, L.; Klose, U.; Knipper, M. Reduced sound-evoked and resting-state BOLD fMRI connectivity in tinnitus. Neuroimage Clin. 2018, 20, 637–649. [Google Scholar] [CrossRef]
- Hofmeier, B.; Wertz, J.; Refat, F.; Hinrichs, P.; Saemisch, J.; Singer, W.; Ruttiger, L.; Klose, U.; Knipper, M.; Wolpert, S. Functional biomarkers that distinguish between tinnitus with and without hyperacusis. Clin. Transl. Med. 2021, 11, e378. [Google Scholar] [CrossRef]
- Won, J.H.; Tremblay, K.; Clinard, C.G.; Wright, R.A.; Sagi, E.; Svirsky, M. The neural encoding of formant frequencies contributing to vowel identification in normal-hearing listeners. J. Acoust. Soc. Am. 2016, 139, 1–11. [Google Scholar] [CrossRef]
- Hornickel, J.; Skoe, E.; Nicol, T.; Zecker, S.; Kraus, N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc. Natl. Acad. Sci. USA 2009, 106, 13022–13027. [Google Scholar] [CrossRef]
- Verschooten, E.; Shamma, S.; Oxenham, A.J.; Moore, B.C.J.; Joris, P.X.; Heinz, M.G.; Plack, C.J. The upper frequency limit for the use of phase locking to code temporal fine structure in humans: A compilation of viewpoints. Hear. Res. 2019, 377, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Weiss, T.F.; Rose, C. A comparison of synchronization filters in different auditory receptor organs. Hear. Res. 1988, 33, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Desmadryl, G.; Justal, T.; Nouvian, R.; Puel, J.L.; Bourien, J. The Interplay Between Spike-Time and Spike-Rate Modes in the Auditory Nerve Encodes Tone-In-Noise Threshold. J. Neurosci. 2018, 38, 5727–5738. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.C.J. Effects of hearing loss and age on the binaural processing of temporal envelope and temporal fine structure information. Hear. Res. 2021, 402, 107991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Isik, M.; Borst, A.; Hemmert, W. Auditory information coding by modeled cochlear nucleus neurons. J. Comput. Neurosci. 2011, 30, 529–542. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Maele, T.V.; Keshishzadeh, S.; Poortere, N.; Dhooge, I.; Keppler, H.; Verhulst, S. The Variability in Potential Biomarkers for Cochlear Synaptopathy After Recreational Noise Exposure. J. Speech Lang Hear. Res. 2021, 64, 4964–4981. [Google Scholar] [CrossRef] [PubMed]
- Shanks, J.E. Tympanometry. Ear Hear. 1984, 5, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.H.; Goycoolea, H.G. Multifrequency tympanometry in normal adults. Ear Hear. 1993, 14, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Vasilkov, V.; Garrett, M.; Mauermann, M.; Verhulst, S. Enhancing the sensitivity of the envelope-following response for cochlear synaptopathy screening in humans: The role of stimulus envelope. Hear. Res. 2021, 400, 108132. [Google Scholar] [CrossRef] [PubMed]
- Shera, C.A.; Guinan, J.J., Jr. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 1999, 105, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.; Gummer, A.W.; Dalhoff, E. Extraction of distortion-product otoacoustic emission source components and its relevance for objective audiometry. Procedia IUTAM 2016, 100, 38–47. [Google Scholar] [CrossRef]
- Kummer, P.; Janssen, T.; Arnold, W. The level and growth behavior of the 2 f1-f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss. J. Acoust. Soc. Am. 1998, 103, 3431–3444. [Google Scholar] [CrossRef] [PubMed]
- Boege, P.; Janssen, T. Pure-tone threshold estimation from extrapolated distortion product otoacoustic emission I/O-functions in normal and cochlear hearing loss ears. J. Acoust. Soc. Am. 2002, 111, 1810–1818. [Google Scholar] [CrossRef]
- Gorga, M.P.; Neely, S.T.; Dorn, P.A.; Hoover, B.M. Further efforts to predict pure-tone thresholds from distortion product otoacoustic emission input/output functions. J. Acoust. Soc. Am. 2003, 113, 3275–3284. [Google Scholar] [CrossRef]
- Johnson, T.A.; Neely, S.T.; Kopun, J.G.; Dierking, D.M.; Tan, H.; Converse, C.; Kennedy, E.; Gorga, M.P. Distortion product otoacoustic emissions: Cochlear-source contributions and clinical test performance. J. Acoust. Soc. Am. 2007, 122, 3539–3553. [Google Scholar] [CrossRef]
- Wagener, K.; Kühnel, V.; Kollmeier, B. Development and evaluation of a German sentence test I: Design of the Oldenburg sentence test. Z. Audiol. 1999, 38, 4–15. [Google Scholar]
- Brand, T.; Kollmeier, B. Efficient adaptive procedures for threshold and concurrent slope estimates for psychophysics and speech intelligibility tests. J. Acoust. Soc. Am. 2002, 111, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.C.; Brand, T.; Kollmeier, B. Development and evaluation of a German sentence test Part III: Evaluation of the Oldenburg sentence test. Z. Audiol. 1999, 38, 86–95. [Google Scholar]
- Wagener, K.C.; Brand, T. Sentence intelligibility in noise for listeners with normal hearing and hearing impairment: Influence of measurement procedure and masking parameters. Int. J. Audiol. 2005, 44, 144–156. [Google Scholar] [CrossRef]
- Byrne, D.; Dillon, H.; Tran, K.; Arlinger, S.; Wilbraham, K.; Cox, R.; Hagerman, B.; Hetu, R.; Kei, J.; Lui, C.; et al. An international comparison of long-term average speech spectra. J. Acoust. Soc. Am. 1994, 96, 2108–2120. [Google Scholar] [CrossRef]
- Morise, M.; Yokomori, F.; Ozawa, K. WORLD: A Vocoder-Based High-Quality Speech Synthesis System for Real-Time Applications. IEICE Trans. Inf. Syst. 2016, E99, 1877–1884. [Google Scholar] [CrossRef]
- Marcher-Rorsted, J.; Encina-Llamas, G.; Dau, T.; Liberman, M.C.; Wu, P.Z.; Hjortkjaer, J. Age-related reduction in frequency-following responses as a potential marker of cochlear neural degeneration. Hear. Res. 2022, 414, 108411. [Google Scholar] [CrossRef]
- Carcagno, S.; Plack, C.J. Relations between speech-reception, psychophysical temporal processing, and subcortical electrophysiological measures of auditory function in humans. Hear. Res. 2022, 417, 108456. [Google Scholar] [CrossRef] [PubMed]
- Osterhammel, D.; Osterhammel, P. High-frequency audiometry. Age and sex variations. Scand. Audiol. 1979, 8, 73–81. [Google Scholar] [CrossRef]
- Prendergast, G.; Hymers, M.; Lee, A. A quick and reliable estimate of extended high-frequency hearing. Int. J. Audiol. 2020, 59, 823–827. [Google Scholar] [CrossRef]
- Wardenga, N.; Batsoulis, C.; Wagener, K.C.; Brand, T.; Lenarz, T.; Maier, H. Do you hear the noise? The German matrix sentence test with a fixed noise level in subjects with normal hearing and hearing impairment. Int. J. Audiol. 2015, 54 (Suppl. S2), 71–79. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.X.; Schreiner, C.E.; Rees, A. Neural processing of amplitude-modulated sounds. Physiol. Rev. 2004, 84, 541–577. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Odenthal, D.W. Action potentials and summating potentials in the normal human cochlea. Acta Otolaryngol. Suppl. 1974, 316, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Z.; Liberman, M.C. Age-related stereocilia pathology in the human cochlea. Hear. Res. 2022, 422, 108551. [Google Scholar] [CrossRef]
- Olson, H.F. Frequency Range Preference for Speech and Music. J. Acoust. Soc. Am. 1947, 19, 549–555. [Google Scholar] [CrossRef]
- Moore, B.C. Basic auditory processes involved in the analysis of speech sounds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Buran, B.N.; Strenzke, N.; Neef, A.; Gundelfinger, E.D.; Moser, T.; Liberman, M.C. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J. Neurosci. 2010, 30, 7587–7597. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Kiang, N.Y. Analysis of discharges recorded simultaneously from pairs of auditory nerve fibers. Biophys. J. 1976, 16, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Rhode, W.S.; Smith, P.H. Encoding timing and intensity in the ventral cochlear nucleus of the cat. J. Neurophysiol. 1986, 56, 261–286. [Google Scholar] [CrossRef]
- Zohar, O.; Shackleton, T.M.; Nelken, I.; Palmer, A.R.; Shamir, M. First spike latency code for interaural phase difference discrimination in the guinea pig inferior colliculus. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 9192–9204. [Google Scholar] [CrossRef]
- Meddis, R. Auditory-nerve first-spike latency and auditory absolute threshold: A computer model. J. Acoust. Soc. Am. 2006, 119, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Heil, P.; Neubauer, H.; Brown, M.; Irvine, D.R. Towards a unifying basis of auditory thresholds: Distributions of the first-spike latencies of auditory-nerve fibers. Hear. Res. 2008, 238, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Batrel, C.; Wang, J.; Desmadryl, G.; Nouvian, R.; Puel, J.L.; Bourien, J. Sound Coding in the Auditory Nerve: From Single Fiber Activity to Cochlear Mass Potentials in Gerbils. Neuroscience 2019, 407, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bramhall, N.F. Use of the auditory brainstem response for assessment of cochlear synaptopathy in humans. J. Acoust. Soc. Am. 2021, 150, 4440. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Kuwada, S.; Maher, V.L. The frequency-following response to continuous tones in humans. Hear. Res. 1986, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Parbery-Clark, A.; White-Schwoch, T.; Kraus, N. Aging affects neural precision of speech encoding. J. Neurosci. 2012, 32, 14156–14164. [Google Scholar] [CrossRef] [PubMed]
- Frisina, D.R.; Frisina, R.D. Speech recognition in noise and presbycusis: Relations to possible neural mechanisms. Hear. Res. 1997, 106, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984, 16, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Motlagh Zadeh, L.; Silbert, N.H.; Sternasty, K.; Swanepoel, W.; Hunter, L.L.; Moore, D.R. Extended high-frequency hearing enhances speech perception in noise. Proc. Natl. Acad. Sci. USA 2019, 116, 23753–23759. [Google Scholar] [CrossRef]
- Shehabi, A.M.; Prendergast, G.; Plack, C.J. The Relative and Combined Effects of Noise Exposure and Aging on Auditory Peripheral Neural Deafferentation: A Narrative Review. Front. Aging Neurosci. 2022, 14, 877588. [Google Scholar] [CrossRef]
- Boettcher, F.A.; Poth, E.A.; Mills, J.H.; Dubno, J.R. The amplitude-modulation following response in young and aged human subjects. Hear. Res. 2001, 153, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Rumschlag, J.A.; Razak, K.A. Age-related changes in event related potentials, steady state responses and temporal processing in the auditory cortex of mice with severe or mild hearing loss. Hear. Res. 2021, 412, 108380. [Google Scholar] [CrossRef] [PubMed]
- Menard, M.; Gallego, S.; Berger-Vachon, C.; Collet, L.; Thai-Van, H. Relationship between loudness growth function and auditory steady-state response in normal-hearing subjects. Hear. Res. 2008, 235, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, A.J. How We Hear: The Perception and Neural Coding of Sound. Annu. Rev. Psychol. 2018, 69, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Abdala, C.; Ortmann, A.J.; Guardia, Y.C. Weakened Cochlear Nonlinearity During Human Aging and Perceptual Correlates. Ear Hear. 2021, 42, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Kiang, N.Y.; Watanabe, T.; Thomas, E.C.; Clark, L.F. Discharge Patterns of Single Fibers in the Cat’s Auditory Nerve; M.I.T. Press: Oxford, UK, 1966; p. xvii, 154. [Google Scholar]
- Kim, D.O.; Dorn, P.A.; Neely, S.T.; Gorga, M.P. Adaptation of distortion product otoacoustic emission in humans. J. Assoc. Res. Otolaryngol. 2001, 2, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bassim, M.K.; Miller, R.L.; Buss, E.; Smith, D.W. Rapid adaptation of the 2f1-f2 DPOAE in humans: Binaural and contralateral stimulation effects. Hear. Res. 2003, 182, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Goutman, J.D. Mechanisms of synaptic depression at the hair cell ribbon synapse that support auditory nerve function. Proc. Natl. Acad. Sci. USA 2017, 114, 9719–9724. [Google Scholar] [CrossRef]
- Moser, T.; Beutner, D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 883–888. [Google Scholar] [CrossRef]
- Peterson, A.J.; Huet, A.; Bourien, J.; Puel, J.L.; Heil, P. Recovery of auditory-nerve-fiber spike amplitude under natural excitation conditions. Hear. Res. 2018, 370, 248–263. [Google Scholar] [CrossRef]
- Willmore, B.D.B.; King, A.J. Adaptation in auditory processing. Physiol. Rev. 2023, 103, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Ito, T.; Abe, Y.; Chiba, H.; Suzuki, Y.; Kakehata, S.; Aoyagi, M. Detecting the recruitment phenomenon in adults using 80-Hz auditory steady-state response. Auris Nasus Larynx 2019, 46, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Denes, P.; Naunton, R.F. The clinical detection of auditory recruitment. J. Laryngol. Otol. 1950, 64, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Rasetshwane, D.M.; Argenyi, M.; Neely, S.T.; Kopun, J.G.; Gorga, M.P. Latency of tone-burst-evoked auditory brain stem responses and otoacoustic emissions: Level, frequency, and rise-time effects. J. Acoust. Soc. Am. 2013, 133, 2803–2817. [Google Scholar] [CrossRef] [PubMed]
- Suthakar, K.; Liberman, M.C. Noise Masking in Cochlear Synaptopathy: Auditory Brainstem Response vs. Auditory Nerve Response in Mouse. J. Neurophysiol. 2022, 127, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, A.J. Predicting the Perceptual Consequences of Hidden Hearing Loss. Trends Hear. 2016, 20, 2331216516686768. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.H. Supra-Threshold Hearing and Fluctuation Profiles: Implications for Sensorineural and Hidden Hearing Loss. J. Assoc. Res. Otolaryngol. 2018, 19, 331–352. [Google Scholar] [CrossRef] [PubMed]
- Chimento, T.C.; Schreiner, C.E. Adaptation and recovery from adaptation in single fiber responses of the cat auditory nerve. J. Acoust. Soc. Am. 1991, 90, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Sachs, M.B. Recovery from sound exposure in auditory-nerve fibers. J. Acoust. Soc. Am. 1973, 54, 1535–1543. [Google Scholar] [CrossRef]
- Huet, A.; Batrel, C.; Dubernard, X.; Kleiber, J.C.; Desmadryl, G.; Venail, F.; Liberman, M.C.; Nouvian, R.; Puel, J.L.; Bourien, J. Peristimulus Time Responses Predict Adaptation and Spontaneous Firing of Auditory-Nerve Fibers: From Rodents Data to Humans. J. Neurosci. 2022, 42, 2253–2267. [Google Scholar] [CrossRef]
- Bourien, J.; Tang, Y.; Batrel, C.; Huet, A.; Lenoir, M.; Ladrech, S.; Desmadryl, G.; Nouvian, R.; Puel, J.L.; Wang, J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J. Neurophysiol. 2014, 112, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.; Bader, K.; Dierkes, L.; Gummer, A.W.; Dalhoff, E. Derivation of input-output functions from distortion-product otoacoustic emission level maps. J. Acoust. Soc. Am. 2020, 147, 3169–3187. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.; Dierkes, L.; Braun, L.H.; Gummer, A.W.; Dalhoff, E.; Zelle, D. Test-retest reliability of distortion-product thresholds compared to behavioral auditory thresholds. Hear. Res. 2021, 406, 108232. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, M.L.; Stagner, B.B.; Martin, G.K.; Lonsbury-Martin, B.L. Visualization of the onset of distortion-product otoacoustic emissions, and measurement of their latency. J. Acoust. Soc. Am. 1996, 100, 1663–1679. [Google Scholar] [CrossRef]
- Krokenberger, M. Adaptive DPOAE-Wachstumsfunktionen zur Objektiven Hörschwellenschätzung bei Normalhörenden und Hörgeschädigten Ohren; Universität Tübingen: Tübingen, Germany, 2019. [Google Scholar]
- Zelle, D.; Gummer, A.W.; Dalhoff, E. Extraction of otoacoustic distortion product sources using pulse basis functions. J. Acoust. Soc. Am. 2013, 134, EL64–EL69. [Google Scholar] [CrossRef]










| Noise Conditions | PNOT | n | Age Mean ± SEM | PTA4 Mean ± SEM | PTA-EHF Mean ± SEM |
|---|---|---|---|---|---|
| In quiet | good | 30 | 45.40 ± 2.87 | 12.33 ± 1.21 | 36.22 ± 3.24 |
| standard | 29 | 38.48 ± 3.10 | 13.41 ± 1.44 | 28.67 ± 3.73 | |
| poor | 30 | 47.60 ± 3.25 | 13.40 ± 1.18 | 38.28 ± 3.73 | |
| In ipsilateral noise | good | 21 | 45.24 ± 3.64 | 14.02 ± 1.49 | 36.00 ± 4.19 |
| standard | 21 | 41.05 ± 3.87 | 13.21 ± 1.53 | 29.42 ± 4.67 | |
| poor | 21 | 42.10 ± 3.70 | 12.38 ± 1.78 | 34.30 ± 4.25 |
| R2 | p | n | ||
|---|---|---|---|---|
| OLSA quiet | BB over PTA4 | 0.5363 | <0.00001 | 89 |
| LP over PTA-LF | 0.4574 | <0.00001 | 89 | |
| HP over PTA-LF | 0.0388 | 0.064394 | 89 | |
| HP over PTA-EHF | 0.378 | <0.00001 | 89 | |
| OLSA ipsilateral noise | BB over PTA4 | 0.3111 | <0.00001 | 63 |
| LP over PTA-LF | 0.064 | 0.045525 | 63 | |
| HP over PTA-HF | 0.1826 | 0.000478 | 63 | |
| HP over PTA-EHF | 0.1748 | 0.00065 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirmer, J.; Wolpert, S.; Dapper, K.; Rühle, M.; Wertz, J.; Wouters, M.; Eldh, T.; Bader, K.; Singer, W.; Gaudrain, E.; et al. Neural Adaptation at Stimulus Onset and Speed of Neural Processing as Critical Contributors to Speech Comprehension Independent of Hearing Threshold or Age. J. Clin. Med. 2024, 13, 2725. https://doi.org/10.3390/jcm13092725
Schirmer J, Wolpert S, Dapper K, Rühle M, Wertz J, Wouters M, Eldh T, Bader K, Singer W, Gaudrain E, et al. Neural Adaptation at Stimulus Onset and Speed of Neural Processing as Critical Contributors to Speech Comprehension Independent of Hearing Threshold or Age. Journal of Clinical Medicine. 2024; 13(9):2725. https://doi.org/10.3390/jcm13092725
Chicago/Turabian StyleSchirmer, Jakob, Stephan Wolpert, Konrad Dapper, Moritz Rühle, Jakob Wertz, Marjoleen Wouters, Therese Eldh, Katharina Bader, Wibke Singer, Etienne Gaudrain, and et al. 2024. "Neural Adaptation at Stimulus Onset and Speed of Neural Processing as Critical Contributors to Speech Comprehension Independent of Hearing Threshold or Age" Journal of Clinical Medicine 13, no. 9: 2725. https://doi.org/10.3390/jcm13092725