Chromatographic Assessment of Organic Compounds Using Carbon Nanotubes: The Relationship between Affinity and Dispersibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication Procedure of CNT Columns
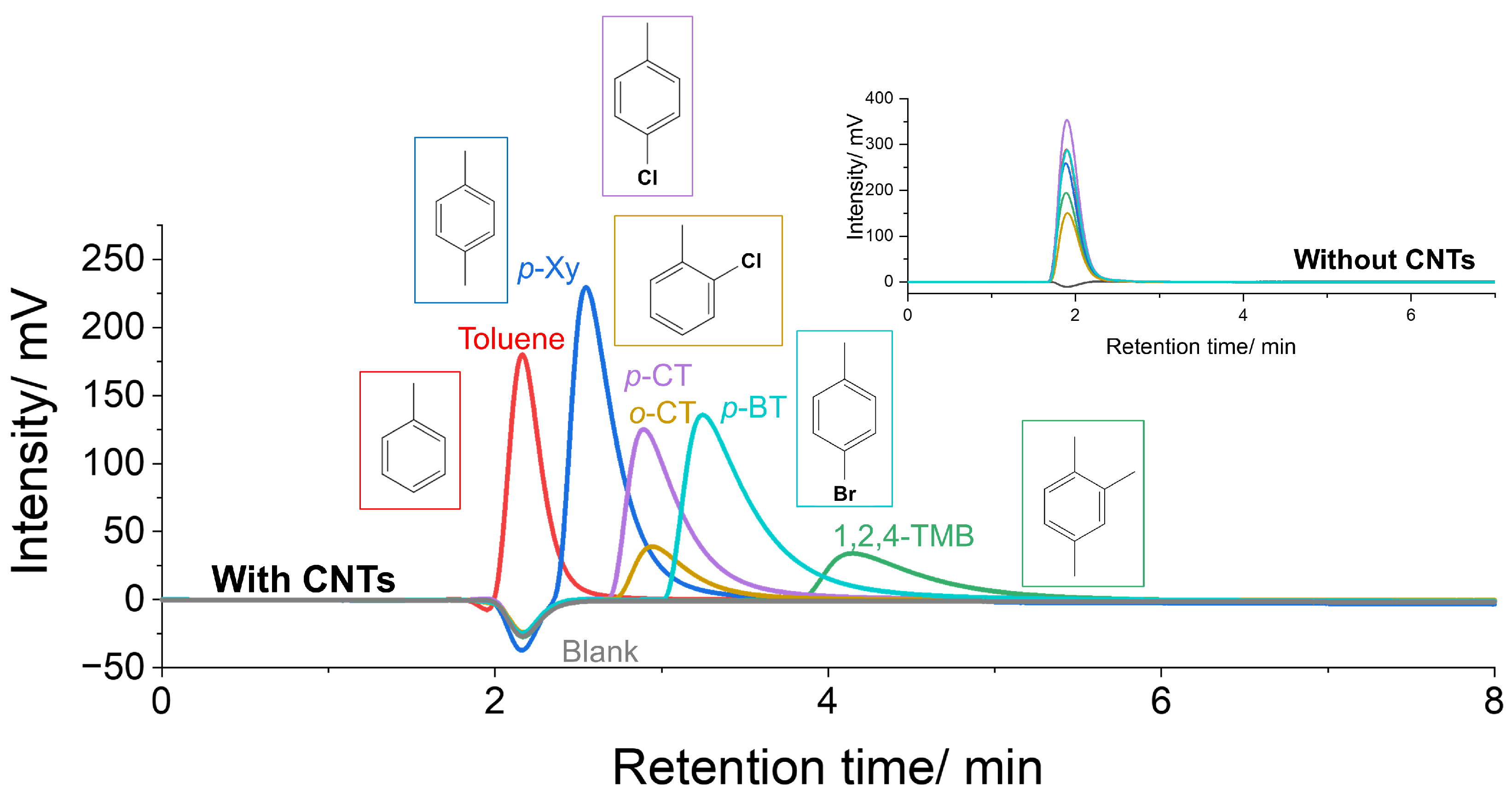
2.3. Chromatographic Analysis
2.4. Evaluation of Dispersibility of CNTs
2.5. Other Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ausman, K.D.; Piner, R.; Lourie, O.; Ruoff, R.S.; Korobov, M. Organic Solvent Dispersions of Single-Walled Carbon Nanotubes: Toward Solutions of Pristine Nanotubes. J. Phys. Chem. B 2000, 104, 8911–8915. [Google Scholar] [CrossRef]
- Choi, J.; Lee, C.; Park, S.; Embleton, T.J.; Ko, K.; Jo, M.; Saqib, K.S.; Yun, J.; Jo, M.; Son, Y.; et al. Analysis of Electrochemical Performance with Dispersion Degree of CNTs in Electrode According to Ultrasonication Process and Slurry Viscosity for Lithium-Ion Battery. Nanomaterials 2022, 12, 4271. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kishi, R.; Kobashi, K.; Morimoto, T.; Okazaki, T.; Yamada, T.; Hata, K. Improved thermal stability of silicone rubber nanocomposites with low filler content, achieved by well-dispersed carbon nanotubes. Compos. Commun. 2020, 22, 100482. [Google Scholar] [CrossRef]
- Hansen, C.M. 50 Years with solubility parameters—Past and future. Prog. Org. Coat. 2004, 51, 77–84. [Google Scholar] [CrossRef]
- Wieneke, J.U.; Kommoß, B.; Gaer, O.; Prykhodko, I.; Ulbricht, M. Systematic Investigation of Dispersions of Unmodified Inorganic Nanoparticles in Organic Solvents with Focus on the Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2011, 51, 327–334. [Google Scholar] [CrossRef]
- Cheng, Q.; Debnath, S.; O’neill, L.; Hedderman, T.G.; Gregan, E.; Byrne, H.J. Systematic Study of the Dispersion of SWNTs in Organic Solvents. J. Phys. Chem. C 2010, 114, 4857–4863. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery. Langmuir 2009, 26, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Zuaznabar-Gardona, J.C.; Fragoso, A. Determination of the Hansen solubility parameters of carbon nano-onions and prediction of their dispersibility in organic solvents. J. Mol. Liq. 2019, 294, 111646. [Google Scholar] [CrossRef]
- Ma, J.; Larsen, R.M. Comparative Study on Dispersion and Interfacial Properties of Single Walled Carbon Nanotube/Polymer Composites Using Hansen Solubility Parameters. ACS Appl. Mater. Interfaces 2013, 5, 1287–1293. [Google Scholar] [CrossRef]
- Bergin, S.D.; Sun, Z.; Rickard, D.; Streich, P.V.; Hamilton, J.P.; Coleman, J.N. Multicomponent Solubility Parameters for Single-Walled Carbon Nanotube−Solvent Mixtures. ACS Nano 2009, 3, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, M.; Cárdenas, S.; Simonet, B.M. Role of Carbon Nanotubes in Analytical Science. Anal. Chem. 2007, 79, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Saridara, C.; Mitra, S. Chromatography on Self-Assembled Carbon Nanotubes. Anal. Chem. 2005, 77, 7094–7097. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xiang, R.; Ciuparu, D.; Pfefferle, L.D.; Horváth, C.; Wilkins, J.A. Incorporation of Single-Wall Carbon Nanotubes into an Organic Polymer Monolithic Stationary Phase for μ-HPLC and Capillary Electrochromatography. Anal. Chem. 2005, 77, 1398–1406. [Google Scholar] [CrossRef]
- Yuan, L.-M.; Ren, C.-X.; Li, L.; Ai, P.; Yan, Z.-H.; Zi, M.; Li, Z.-Y. Single-Walled Carbon Nanotubes Used as Stationary Phase in GC. Anal. Chem. 2006, 78, 6384–6390. [Google Scholar] [CrossRef]
- Chang, Y.X.; Zhou, L.L.; Li, G.X.; Li, L.; Yuan, L.M. Single?Wall Carbon Nanotubes Used as Stationary Phase in HPLC. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2953–2958. [Google Scholar] [CrossRef]
- Yoo, J.; Ozawa, H.; Fujigaya, T.; Nakashima, N. Evaluation of affinity of molecules for carbon nanotubes. Nanoscale 2011, 3, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, K.; Shiraki, K.; Ishii, R.; Tanaka, T.; Hirano, A. Liquid Chromatographic Analysis of the Interaction between Amino Acids and Aromatic Surfaces Using Single-Wall Carbon Nanotubes. Langmuir 2015, 31, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Kanao, E.; Morinaga, T.; Kubo, T.; Naito, T.; Matsumoto, T.; Sano, T.; Maki, H.; Yan, M.; Otsuka, K. Separation of halogenated benzenes enabled by investigation of halogen–π interactions with carbon materials. Chem. Sci. 2019, 11, 409–418. [Google Scholar] [CrossRef]
- Zhang, Y.; McGuffin, V.L. Thermodynamic and Kinetic Characterization of Porous Graphitic Carbon in Reversed-Phase Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1551–1575. [Google Scholar] [CrossRef]
- Takeyama, Y.; Ueno, M. Elastomer Composition, Method for Producing Same, Crosslinked Substance, and Molded Body. WO 2021/172555 A1, 26 February 2021. [Google Scholar]
- Kobashi, K.; Sekiguchi, A.; Yamada, T.; Muroga, S.; Okazaki, T. Dispersions of High-Quality Carbon Nanotubes with Narrow Aggregate Size Distributions by Viscous Liquid for Conducting Polymer Composites. ACS Appl. Nano Mater. 2020, 3, 1391–1399. [Google Scholar] [CrossRef]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen Solubility Parameters with a New Group-Contribution Method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Hirano, A.; Kameda, T. Aromaphilicity Index of Amino Acids: Molecular Dynamics Simulations of the Protein Binding Affinity for Carbon Nanomaterials. ACS Appl. Nano Mater. 2021, 4, 2486–2495. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Lin, D.; Futaba, D.N.; Kobashi, K.; Zhang, M.; Muroga, S.; Chen, G.; Tsuji, T.; Hata, K. A Microwave-Assisted, Solvent-Free Approach for the Versatile Functionalization of Carbon Nanotubes. ACS Nano 2023, 17, 3976–3983. [Google Scholar] [CrossRef]




| Toluene | p-Xylene (p-Xy) | 1,2,4-Trimethylbenzene (1,2,4-TMB) | o-Chlorotoluene (o-CT) | |
|---|---|---|---|---|
| Viscosity/mPa s (20 °C) | 0.59 | 0.6475 | 0.9 | 0.884 (30 °C) |
| Density/g cm−3 | 0.867 | 0.861 | 0.876 | 1.087 |
| CNT height percentage after stirring 1/% | 39 | 41 | 59 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, T.; Kishi, R.; Hirano, A.; Kokubo, K.; Hata, K. Chromatographic Assessment of Organic Compounds Using Carbon Nanotubes: The Relationship between Affinity and Dispersibility. Nanomaterials 2024, 14, 824. https://doi.org/10.3390/nano14100824
Shimizu T, Kishi R, Hirano A, Kokubo K, Hata K. Chromatographic Assessment of Organic Compounds Using Carbon Nanotubes: The Relationship between Affinity and Dispersibility. Nanomaterials. 2024; 14(10):824. https://doi.org/10.3390/nano14100824
Chicago/Turabian StyleShimizu, Taiyo, Ryoichi Kishi, Atsushi Hirano, Ken Kokubo, and Kenji Hata. 2024. "Chromatographic Assessment of Organic Compounds Using Carbon Nanotubes: The Relationship between Affinity and Dispersibility" Nanomaterials 14, no. 10: 824. https://doi.org/10.3390/nano14100824





