Schottky Junctions with Bi@Bi2MoO6 Core-Shell Photocatalysts toward High-Efficiency Solar N2-to-Ammonnia Conversion in Aqueous Phase
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Photocatalysts
2.3. Characterization of Photocatalysts
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic N2 Fixation Reaction
2.6. Determination of Ammonia
3. Results and Discussion
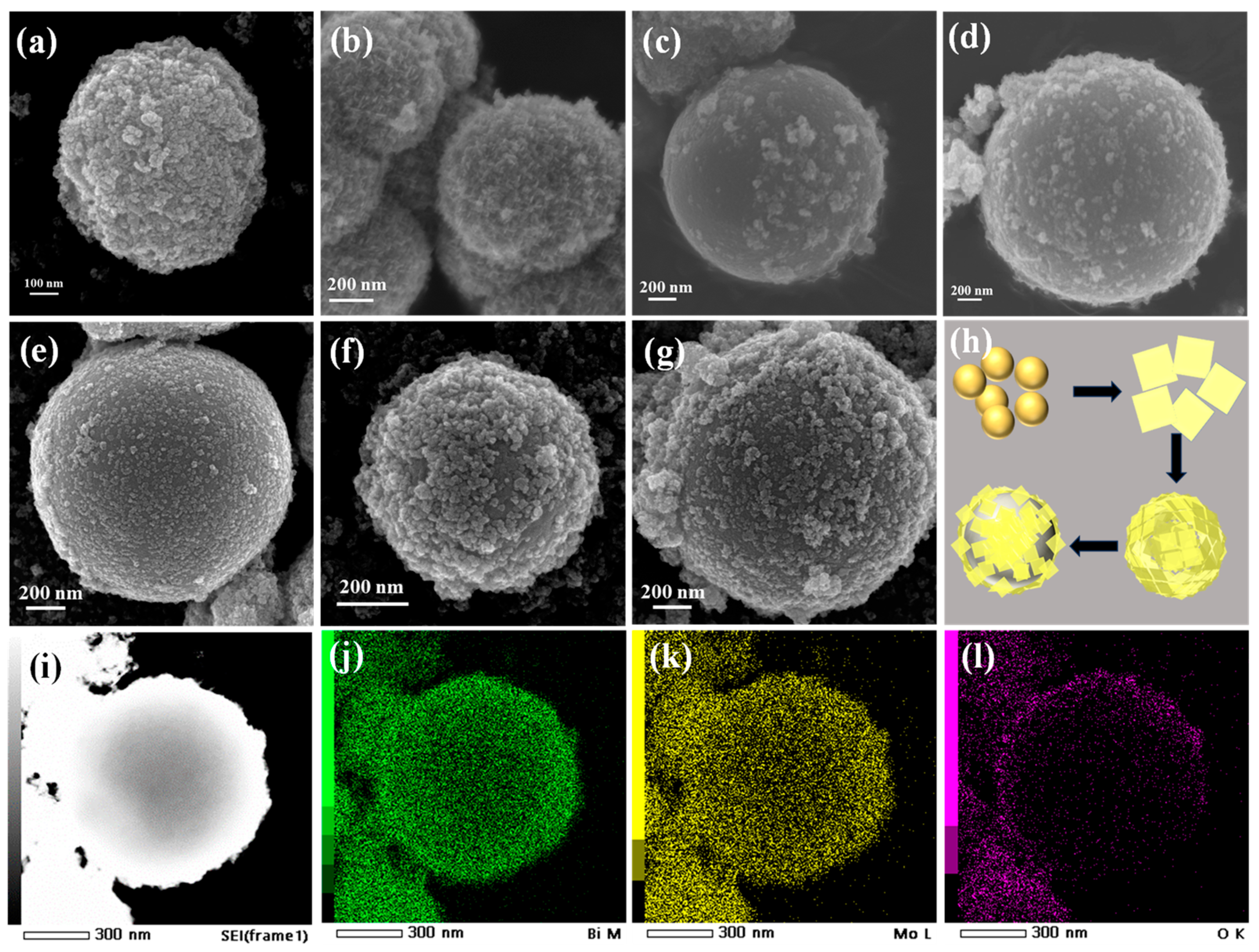
3.1. The Structure of Samples
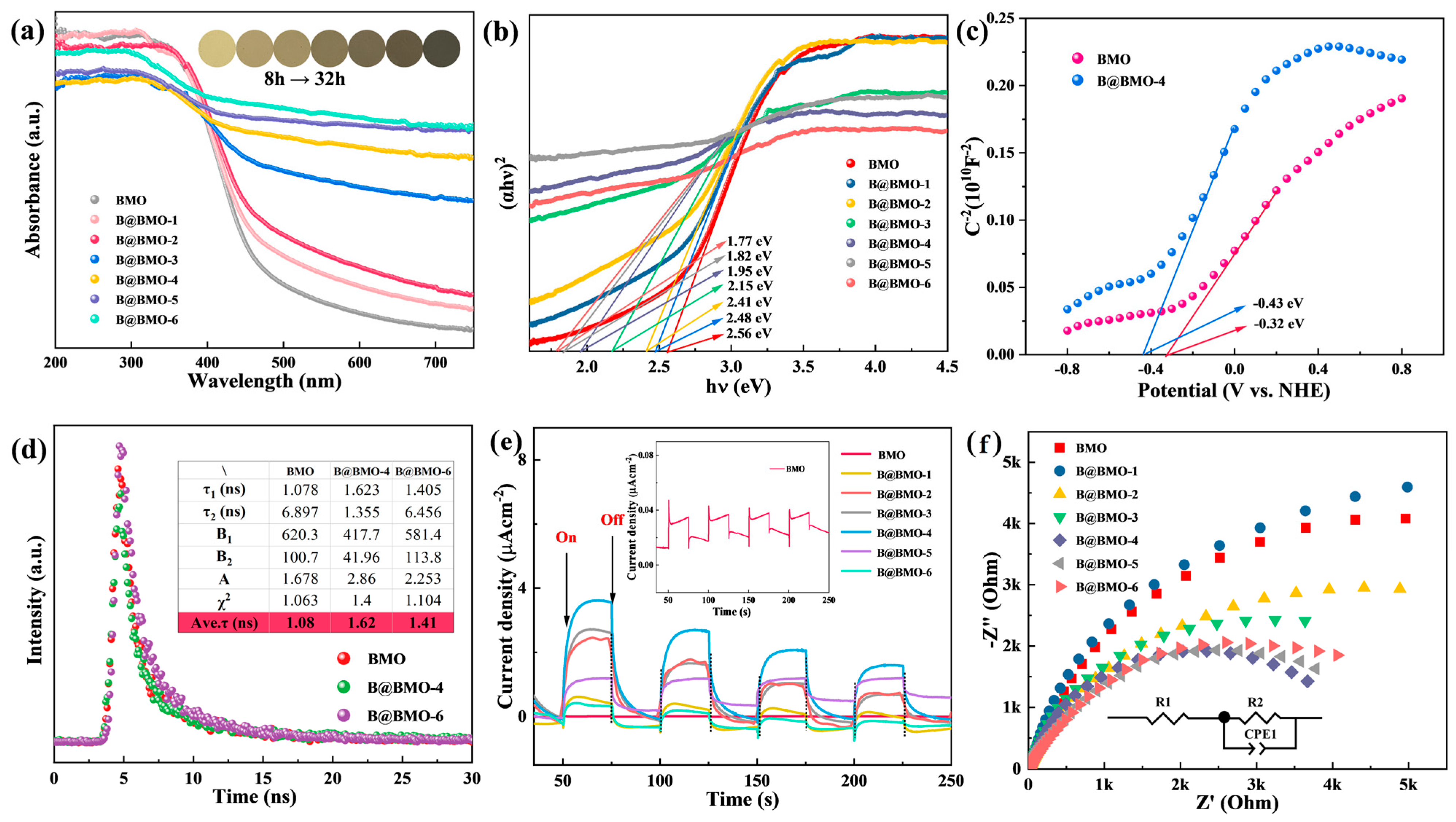
3.2. Photoelectrochemical Properties and Photocatalytic N2 to NH3
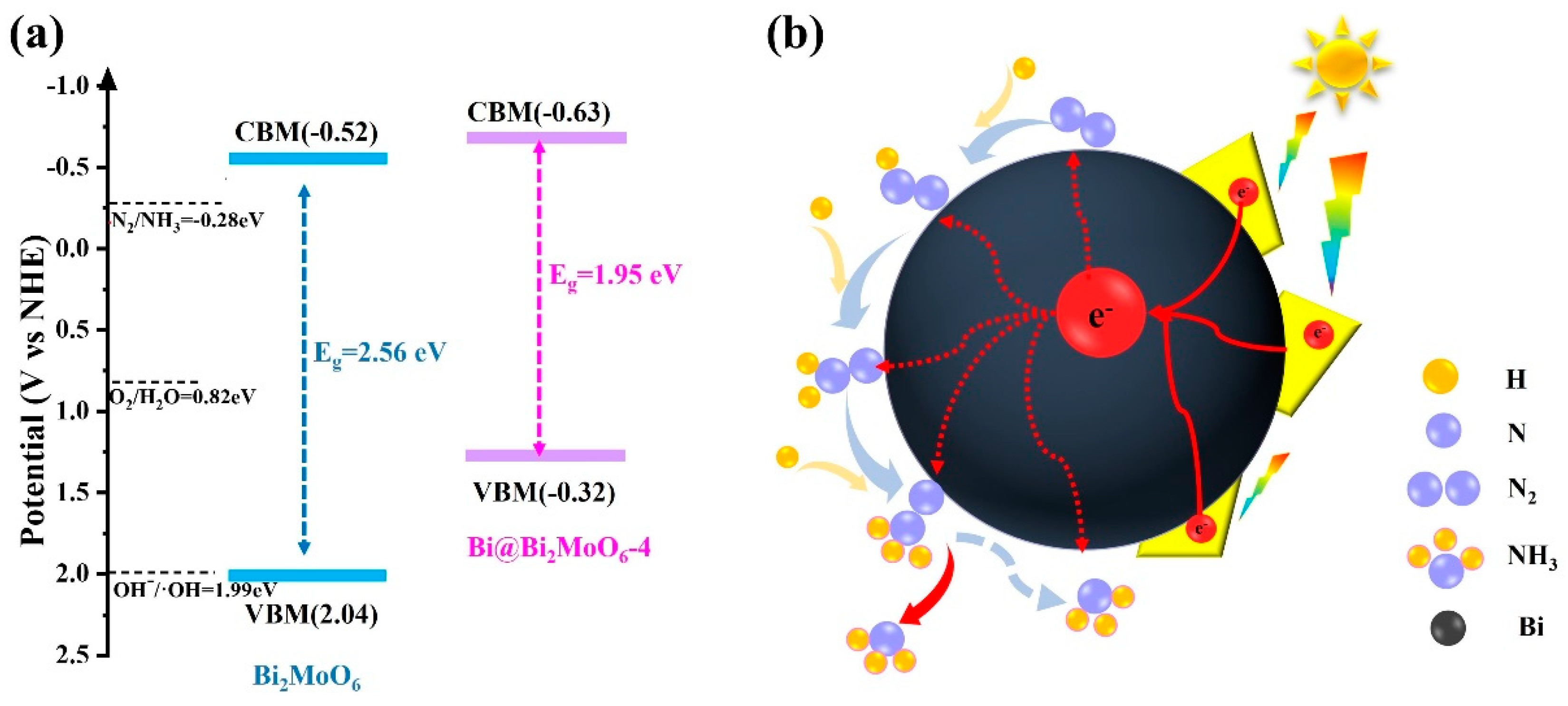
3.3. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Yang, B.; Zhou, Y.; Luo, W.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tungsten Nitride/Tungsten Oxide Nanosheets for Enhanced Oxynitride Intermediate Adsorption and Hydrogenation in Nitrate Electroreduction to Ammonia. ACS Nano 2023, 17, 25091–25100. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Pedersen, J.B.; Zhou, Y.; Saccoccio, M.; Li, S.; Salinas, R.; Li, K.; Andersen, S.Z.; Xu, A.; Deissler, N.H.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wang, Z.; Zhang, N.; Bao, D.; Zhong, H.; Zhang, X. Potential-Induced Synthesis and Structural Identification of Oxide-Derived Cu Electrocatalysts for Selective Nitrate Reduction to Ammonia. ACS Catal. 2023, 13, 7529–7537. [Google Scholar] [CrossRef]
- Kisch, H. In the Light and in the Dark: Photocatalytic Fixation of Nitrogen into Ammonia and Nitrate at Iron Titanate Semiconductor Thin Films. Eur. J. Inorg. Chem. 2020, 2020, 1376–1382. [Google Scholar] [CrossRef]
- Wang, T.; Abild-Pedersen, F. Achieving industrial ammonia synthesis rates at near-ambient conditions through modified scaling relations on a confined dual site. Proc. Natl. Acad. Sci. USA 2021, 118, e2106527118. [Google Scholar] [CrossRef] [PubMed]
- Reichle, S.; Felderhoff, M.; Schuth, F. Mechanocatalytic Room-Temperature Synthesis of Ammonia from Its Elements Down to Atmospheric Pressure. Angew. Chem. Int. Ed. 2021, 60, 26385–26389. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Ji, H.; Zhang, L.; Ni, J.; Li, N.; Qian, T.; Yan, C.; Lu, J. Solvent-in-Gas System for Promoted Photocatalytic Ammonia Synthesis on Porous Framework Materials. Adv. Mater. 2023, 35, 202211730. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, J.; Zhao, Z.; Li, Z.; Wang, L.; Zhang, Z.; Li, C.; Meng, X. Defects-Induced Single-Atom Anchoring on Metal-Organic Frameworks for High-Efficiency Photocatalytic Nitrogen Reduction. Angew. Chem. Int. Ed. 2024, 63, 202314408. [Google Scholar] [CrossRef]
- Wu, R.; Gao, S.; Jones, C.; Sun, M.; Guo, M.; Tai, R.; Chen, S.; Wang, Q. Bi/BSO Heterojunctions via Vacancy Engineering for Efficient Photocatalytic Nitrogen Fixation. Adv. Funct. Mater. 2024, 2314051. [Google Scholar] [CrossRef]
- Linnik, O.; Kisch, H. On the mechanism of nitrogen photofixation at nanostructured iron titanate films. Photochem. Photobiol. Sci. 2006, 5, 938–942. [Google Scholar] [CrossRef]
- Yang, H.; Ren, G.; Li, Z.; Zhang, Z.; Meng, X. Fast Joule heating for transformation of Fe-MIL-125(Ti) to Fe/TiO2 with enhanced photocatalytic activity in N2 fixation. Appl. Catal. B-Environ. Energy 2024, 347, 123795. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Luo, S.; Luo, Z.; Zhang, X.; Huang, R.; Luo, M. In situ modification of cobalt on MXene/TiO2 as composite photocatalyst for efficient nitrogen fixation. J. Colloid Interface Sci. 2021, 585, 20–29. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Liu, Q.; Bai, W.; Li, H.; Wang, M.; Qian, Q.; Yang, Q.; Xiao, C.; Xie, Y. Directional Shunting of Photogenerated Carriers in POM@MOF for Promoting Nitrogen Adsorption and Oxidation. Adv. Mater. 2023, 35, 202304532. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Guo, Y.; Zhang, H.; Jiang, R.; Yang, B.; Wang, J.; Yu, J.C. Photodriven Disproportionation of Nitrogen and Its Change to Reductive Nitrogen Photofixation. Angew. Chem. Int. Ed. 2020, 60, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, M.; Sheoran, S.; Jaiswal, S.; Patra, A.; Bhattacharya, S.; Krishnan, V. Tailoring defects in SrTiO3 by one step nanoarchitectonics for realizing photocatalytic nitrogen fixation in pure water. Nanoscale 2023, 15, 11667–11680. [Google Scholar] [CrossRef]
- Pournemati, K.; Habibi-Yangjeh, A.; Khataee, A. TiO2 Quantum Dots/Fe3S4 Heterojunction Nanocomposites for Efficient Ammonia Production through Nitrogen Fixation upon Simulated Sunlight. ACS Appl. Nano Mater. 2024, 7, 2200–2213. [Google Scholar] [CrossRef]
- Vu, V.T.; Vu, T.T.H.; Phan, T.L.; Kang, W.T.; Kim, Y.R.; Tran, M.D.; Nguyen, H.T.T.; Lee, Y.H.; Yu, W.J. One-Step Synthesis of NbSe2/Nb-Doped-WSe2 Metal/Doped-Semiconductor van der Waals Heterostructures for Doping Controlled Ohmic Contact. ACS Nano 2021, 15, 13031–13040. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Xiao, C.; Xie, Y. Dual-Plasmon Resonance Coupling Promoting Directional Photosynthesis of Nitrate from Air. Angew. Chem. Int. Ed. 2023, 62, 202311911. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, M.; He, Z.; Gu, B.; Xiao, C.; Zhou, T.; Guo, Z.; Liu, J.; He, H.; Ye, B.; et al. Pothole-rich Ultrathin WO3 Nanosheets that Trigger N≡N Bond Activation of Nitrogen for Direct Nitrate Photosynthesis. Angew. Chem. Int. Ed. 2019, 58, 731–735. [Google Scholar] [CrossRef]
- Ren, W.; Mei, Z.; Zheng, S.; Li, S.; Zhu, Y.; Zheng, J.; Lin, Y.; Chen, H.; Gu, M.; Pan, F. Wavelength-Dependent Solar N2 Fixation into Ammonia and Nitrate in Pure Water. Research 2020, 2020, 3750314. [Google Scholar] [CrossRef]
- Xia, P.; Pan, X.; Jiang, S.; Yu, J.; He, B.; Ismail, P.M.; Bai, W.; Yang, J.; Yang, L.; Zhang, H.; et al. Designing a Redox Heterojunction for Photocatalytic “Overall Nitrogen Fixation” under Mild Conditions. Adv. Mater. 2022, 34, 202200563. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, Y.; Chen, S.; Xie, X.; Wu, Z.; Zhang, N. Schottky Junctions with Bi Cocatalyst for Taming Aqueous Phase N2 Reduction toward Enhanced Solar Ammonia Production. Adv. Sci. 2021, 8, 202003626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, P.; Zhao, J.; Liu, Y.; Huang, Y.; Huang, H.; Yang, C.; Zhao, Y.; Wu, K.; Fu, X.; et al. The Hole-Tunneling Heterojunction of Hematite-Based Photoanodes Accelerates Photosynthetic Reaction. Angew. Chem. Int. Ed. 2021, 60, 16009–16018. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huo, T.; Deng, Q.; Yu, F.; Xia, Y.; Li, H.; Hou, W. Surface-layer bromine doping enhanced generation of surface oxygen vacancies in bismuth molybdate for efficient photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2022, 310, 121319. [Google Scholar] [CrossRef]
- Xue, X.; Chen, R.; Yan, C.; Hu, Y.; Zhang, W.; Yang, S.; Ma, L.; Zhu, G.; Jin, Z. Efficient photocatalytic nitrogen fixation under ambient conditions enabled by the heterojunctions of n-type Bi2MoO6 and oxygen-vacancy-rich p-type BiOBr. Nanoscale 2019, 11, 10439–10445. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Chen, Y.N. The surface plasmon resonance effect on the enhancement of photodegradation activity by Au/ZnSn(OH)6 nanocubes. Dalton Trans. 2015, 44, 16294–16303. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shen, H.; Guo, L.; Wang, C.; Fu, F.; Liang, Y. Ag/Bi2MoO6-x with enhanced visible-light-responsive photocatalytic activities via the synergistic effect of surface oxygen vacancies and surface plasmon. Appl. Surf. Sci. 2018, 436, 536–547. [Google Scholar] [CrossRef]
- Dong, X.; Xu, L.; Ma, J.; Li, Y.; Yin, Z.; Chen, D.; Wang, Q.; Han, J.; Qiu, J.; Yang, Z.; et al. Enhanced interfacial charge transfer and photothermal effect via in-situ construction of atom co-sharing Bi plasmonic/Bi4O5Br2 nanosheet heterojunction towards improved full-spectrum photocatalysis. Chem. Eng. J. 2023, 459, 141557. [Google Scholar] [CrossRef]
- Fang, M.; Tan, X.; Liu, Z.; Hu, B.; Wang, X. Recent Progress on Metal-Enhanced Photocatalysis: A Review on the Mechanism. Research 2021, 2021, 9794329. [Google Scholar] [CrossRef]
- He, W.; Xiong, J.; Tang, Z.; Wang, Y.; Wang, X.; Xu, H.; Zhao, Z.; Liu, J.; Wei, Y. Localized surface plasmon resonance effect of bismuth nanoparticles in Bi/TiO2 catalysts for boosting visible light-driven CO2 reduction to CH4. Appl. Catal. B-Environ. Energy 2024, 344, 123651. [Google Scholar] [CrossRef]
- Zhao, D.; Xuan, Y.; Zhang, K.; Liu, X. Highly Selective Production of Ethanol over Hierarchical Bi@Bi2MoO6 Composite via Bicarbonate-Assisted Photocatalytic CO2 Reduction. Chemsuschem 2021, 14, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, W.; Sun, Y.; Yu, J.; Zhang, Y.; Wang, H.; Dong, F.; Wu, Z. Bi Cocatalyst/Bi2MoO6 Microspheres Nanohybrid with SPR-Promoted Visible-Light Photocatalysis. J. Phys. Chem. C 2016, 120, 11889–11898. [Google Scholar] [CrossRef]
- Xu, F.; Wang, J.; Zhang, N.; Liang, H.; Sun, H. Simultaneously generating Bi quantum dot and oxygen vacancy on Bi2MoO6 nanosheets for boosting photocatalytic selective alcohol oxidation. Appl. Surf. Sci. 2022, 575, 151738. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.B.; Norskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- Kok, S.H.W.; Lee, J.; Chong, W.K.; Ng, B.-J.; Kong, X.Y.; Ong, W.J.; Chai, S.-P.; Tan, L.-L. Bismuth-rich Bi12O17Cl2 nanorods engineered with oxygen vacancy defects for enhanced photocatalytic nitrogen fixation. J. Alloys Compd. 2023, 952, 170015. [Google Scholar] [CrossRef]
- Lan, M.; Zheng, N.; Dong, X.; Ma, H.; Zhang, X. One-step in-situ synthesis of Bi-decorated BiOBr microspheres with abundant oxygen vacancies for enhanced photocatalytic nitrogen fixation properties. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 623, 126744. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Wang, J.; Zhang, J.; Wu, Y.; He, Y. Cu-doped Bi/B2WO6 catalysts for efficient N2 fixation by photocatalysis. Front. Chem. Sci. Eng. 2023, 17, 1412–1422. [Google Scholar] [CrossRef]
- Geng, M.; Peng, Y.; Zhang, Y.; Guo, X.; Yu, F.; Yang, X.; Xie, G.; Dong, W.; Liu, C.; Li, J.; et al. Hierarchical ZnIn2S4: A promising cocatalyst to boost visible-light-driven photocatalytic hydrogen evolution of In(OH)3. Int. J. Hydrogen Energy 2019, 44, 5787–5798. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Liu, G.; Xie, G.; Guo, Y.; Zhang, Y.; Yu, J. An Efficient ZnIn2S4@CuInS2 Core–Shell p–n Heterojunction to Boost Visible-Light Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2020, 124, 5934–5943. [Google Scholar] [CrossRef]
- Ren, X.; Liu, F.; Wu, H.; Lu, Q.; Zhao, J.; Liu, Y.; Zhang, J.; Mao, J.; Wang, J.; Han, X.; et al. Reconstructed Bismuth Oxide through in situ Carbonation by Carbonate-containing Electrolyte for Highly Active Electrocatalytic CO2 Reduction to Formate. Angew. Chem. Int. Ed. 2024, 63, e202316640. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jia, Y.; Wang, L.; Yang, M.; Bi, Y.; Qi, Y. Efficient Charge Separation between Bi and Bi0-Bi2MoO6 for Photoelectrochemical Properties. Chem. Eur. J. 2016, 22, 5844–5848. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, C.; Guo, L.; Soomro, R.A.; Wang, D.; Xu, B.; Fu, F. Refining electronic properties of Bi2MoO6 by In-doping for boosting overall nitrogen fixation via relay catalysis. Appl. Catal. B Environ. 2023, 330, 122643. [Google Scholar] [CrossRef]
- Yu, B.; Wu, Y.; Meng, F.; Wang, Q.; Jia, X.; Wasim Khan, M.; Huang, C.; Zhang, S.; Yang, L.; Wu, H. Formation of hierarchical Bi2MoO6/ln2S3 S-scheme heterojunction with rich oxygen vacancies for boosting photocatalytic CO2 reduction. Chem. Eng. J. 2022, 429, 132456. [Google Scholar] [CrossRef]
- Li, X.; Dong, Q.; Li, F.; Zhu, Q.; Tian, Q.; Tian, L.; Zhu, Y.; Pan, B.; Padervand, M.; Wang, C. Defective Bi@BiOBr/C microrods derived from Bi-MOF for efficient photocatalytic NO abatement: Directional regulation of interfacial charge transfer via carbon-loading. Appl. Catal. B-Environ. Energy 2024, 340, 123238. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Hu, C.; Shi, B. Enhanced photocatalytic performance by the synergy of Bi vacancies and Bi0 in Bi0-Bi2MoO6. Appl. Catal. B-Environ. 2019, 257, 117785. [Google Scholar] [CrossRef]
- Zhu, Q.; Hailili, R.; Xin, Y.; Zhou, Y.; Huang, Y.; Pang, X.; Zhang, K.; Robertson, P.K.J.; Bahnemann, D.W.; Wang, C. Efficient full spectrum responsive photocatalytic NO conversion at Bi2Ti2O7: Co-effect of plasmonic Bi and oxygen vacancies. Appl. Catal. B Environ. 2022, 319, 121888. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Kong, X.; Li, C.; Lin, B.; Dong, F.; Yang, G.; Shao, G.; Xue, C. Carbon Dots Mediated In Situ Confined Growth of Bi Clusters on g-C3N4 Nanomeshes for Boosting Plasma-Assisted Photoreduction of CO2. Small 2022, 18, 202204154. [Google Scholar] [CrossRef]
- Qin, D.; Song, S.; Liu, Y.; Wang, K.; Yang, B.; Zhang, S. Enhanced Electrochemical Nitrate-to-Ammonia Performance of Cobalt Oxide by Protic Ionic Liquid Modification. Angew. Chem. Int. Ed. 2023, 62, 202304935. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, H.; Sun, Y.; Zhang, G.; Zhou, H.; Cao, S.; Liu, S.; Zhang, L.; Li, W.; Zhu, X.; et al. Synergistic Effect of Oxygen Vacancy and High Porosity of Nano MIL-125(Ti) for Enhanced Photocatalytic Nitrogen Fixation. Angew. Chem. Int. Ed. 2023, 63, 202316973. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Li, Y.-H.; Qi, M.-Y.; Li, J.-Y.; Tang, Z.-R.; Zhou, Z.; Xu, Y.-J. Transition metal doping BiOBr nanosheets with oxygen vacancy and exposed {102} facets for visible light nitrogen fixation. Appl. Catal. B Environ. 2021, 281, 119516. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Diarmand-Khalilabad, H.; Ghosh, S. Nitrogen photofixation ability of g-C3N4 nanosheets/Bi2MoO6 heterojunction photocatalyst under visible-light illumination. J. Colloid Interface Sci. 2020, 563, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, A.; Gill, D.; Jaiswal, S.; Patra, A.; Bhattacharya, S.; Krishnan, V. Boosting Photocatalytic Nitrogen Fixation via Nanoarchitectonics Using Oxygen Vacancy Regulation in W-Doped Bi2MoO6 Nanosheets. ACS Appl. Mater. Interfaces 2023, 15, 55765–55778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, M.; Cao, Y.; Meng, L.; Yang, Y.; Li, X. Tuning the electronic properties of Bi2MoO6 by S-doping to boost efficient photocatalytic nitrogen fixation reactions. J. Catal. 2024, 430, 115347. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, L.; Cui, L.; Zhang, Z.; Li, R.; Wang, Y.; Wang, Y.; Fan, C.; Yu, Z.; Liu, J. Fe-Bi dual sites regulation of Bi2O2.33 nanosheets to promote photocatalytic nitrogen fixation activity. J. Colloid Interface Sci. 2024, 661, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Z.; Shang, Y.; Lv, C.; Zhang, X.; Li, F.; Huang, Q.; Liu, X.; Liu, W.; Zhao, L.; et al. Boosting Carrier Separation on a BiOBr/Bi4O5Br2 Direct Z-Scheme Heterojunction for Superior Photocatalytic Nitrogen Fixation. ACS Catal. 2024, 14, 5779–5787. [Google Scholar] [CrossRef]
- Huang, X.; Du, R.; Zhang, Y.; Ren, J.; Yang, Q.; Wang, K.; Ni, Y.; Yao, Y.; Ali Soomro, R.; Guo, L.; et al. Modulating charge oriented accumulation via interfacial chemical-bond on In2O3/Bi2MoO6 heterostructures for photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2024, 664, 33–44. [Google Scholar] [CrossRef]
- Dong, Q.; Li, X.; Sun, J.; Zhu, Y.; Liang, X.; Ren, H.; Labidi, A.; Wang, D.; Li, F.; Wang, C. Regulating concentration of surface oxygen vacancies in Bi2MoO6/Bi-MOF for boosting photocatalytic ammonia synthesis. J. Catal. 2024, 433, 115489. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; Xie, B.; Meng, Y.; Ni, Z.; Xia, S. S vacancies act as a bridge to promote electron injection from Z-scheme heterojunction to nitrogen molecule for photocatalytic ammonia synthesis. Chem. Eng. J. 2022, 433, 133670. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Deng, X.; Wang, Y.; Meng, G.; Liu, R.; Huang, J.; Tu, M.; Xu, C.; Peng, Y.; et al. Structure and Defect Engineering Synergistically Boost High Solar-to-Chemical Conversion Efficiency of Cerium oxide/Au Hollow Nanomushrooms for Nitrogen Photofixation. Angew. Chem.-Int. Ed. 2024, 63, e202316384. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhao, Y.; Zhang, S.; Li, D.; Shi, R.; Zhou, C.; Wu, L.-Z.; Zhang, T. Enhancing the Supply of Activated Hydrogen to Promote Photocatalytic Nitrogen Fixation. ACS Mater. Lett. 2021, 3, 1521–1527. [Google Scholar] [CrossRef]
- Gao, X.; Shang, Y.; Liu, L.; Gao, K. Ag plasmon resonance promoted 2D AgBr-δ-Bi2O3 nanosheets with enhanced photocatalytic ability. J. Alloys Compd. 2019, 803, 565–575. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Jiang, R.; Qin, F.; Zhang, H.; Lu, W.; Wang, J.; Yu, J.C. High-Efficiency “Working-in-Tandem” Nitrogen Photofixation Achieved by Assembling Plasmonic Gold Nanocrystals on Ultrathin Titania Nanosheets. J. Am. Chem. Soc. 2018, 140, 8497–8508. [Google Scholar] [CrossRef]
- Jia, H.; Du, A.; Zhang, H.; Yang, J.; Jiang, R.; Wang, J.; Zhang, C.-y. Site-Selective Growth of Crystalline Ceria with Oxygen Vacancies on Gold Nanocrystals for Near-Infrared Nitrogen Photofixation. J. Am. Chem. Soc. 2019, 141, 5083–5086. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wei, G.; Li, R.; Yu, M.; Liu, G.; Peng, Y. Schottky Junctions with Bi@Bi2MoO6 Core-Shell Photocatalysts toward High-Efficiency Solar N2-to-Ammonnia Conversion in Aqueous Phase. Nanomaterials 2024, 14, 780. https://doi.org/10.3390/nano14090780
Wang M, Wei G, Li R, Yu M, Liu G, Peng Y. Schottky Junctions with Bi@Bi2MoO6 Core-Shell Photocatalysts toward High-Efficiency Solar N2-to-Ammonnia Conversion in Aqueous Phase. Nanomaterials. 2024; 14(9):780. https://doi.org/10.3390/nano14090780
Chicago/Turabian StyleWang, Meijiao, Guosong Wei, Renjie Li, Meng Yu, Guangbo Liu, and Yanhua Peng. 2024. "Schottky Junctions with Bi@Bi2MoO6 Core-Shell Photocatalysts toward High-Efficiency Solar N2-to-Ammonnia Conversion in Aqueous Phase" Nanomaterials 14, no. 9: 780. https://doi.org/10.3390/nano14090780



